Abstract
While alcohol use disorder (AUD) is a highly heritable condition, the basis of AUD in families with a history of alcoholism is difficult to explain by genetic variation alone. Emerging evidence suggests that parental experience prior to conception can impact inheritance of complex behaviors in offspring via non-genomic (epigenetic) mechanisms. For instance, male C57BL/6J (B6) mice exposed to chronic intermittent vapor ethanol (CIE) prior to mating with Strain 129S1/SvImJ ethanol-naïve females produce male offspring with reduced ethanol drinking preference, increased ethanol sensitivity, and increased BDNF expression in the ventral tegmental area (VTA). In the present study, we tested the hypothesis that these intergenerational effects of paternal CIE are reproducible in male offspring on an inbred B6 background. To this end, B6 males were exposed to six weeks of CIE (or room air as a control) before mating with ethanol-naïve B6 females to produce ethanol (E)-sired and control (C)-sired male and female offspring. We observed a sex-specific effect, as E-sired males exhibited decreased two bottle free-choice ethanol drinking preference, increased sensitivity to the anxiolytic effects of ethanol, and increased VTA BDNF expression; no differences were observed in female offspring. These findings confirm and extend our previous results by demonstrating that the effects of paternal preconception ethanol are reproducible using genetically identical, inbred B6 animals.
Keywords: Epigenetics, Chronic Intermittent Vapor Ethanol, Alcohol drinking, Alcohol sensitivity, BDNF, Stress
Introduction
Twin and adoption studies suggest that alcoholism has a heritability of ~50% (Prescott & Kendler, 1999; Young-Wolff, Enoch, & Prescott, 2011; Ystrom, Reichborn-Kjennerud, Aggen, & Kendler, 2011). Indeed, genome wide association studies (GWAS) have identified several DNA variants associated with a family history of alcoholism, suggesting a significant role for genotype in determining risk for AUD. However, despite the abundant evidence that alcoholism is highly heritable, to date, identified genetic variants only account for a small fraction of AUD heritability (Treutlein & Rietschel, 2011). While this may be due to technical and experimental limitations, it is also possible that alternative biological mechanisms may mediate and explain this “missing” AUD heritability.
Remarkably, many recent studies have found that paternal experience in rodents can drive inheritance of complex phenotypes in offspring. For example, exposing male mice to high fat diet, cocaine, or stress prior to conception has intergenerational effects on glucose tolerance, cocaine preference, or stress responsivity, respectively (Chen et al., 2016; Rodgers, Morgan, Bronson, Revello, & Bale, 2013; Vassoler, White, Schmidt, Sadri-Vakili, & Pierce, 2013). The results from paternal exposure studies are provocative as the inherited phenotypes must be mediated through the germline rather than factors such as in utero physiology that are difficult to control with maternal perturbations. Furthermore, as many of these studies have been performed with animals on an identical genetic background, inheritance cannot be due to transmission of DNA variants across generations. Instead, inherited behavioral phenotypes are hypothesized to be governed through epigenetic mechanisms, defined here as environmentally-induced changes to non-genomic factors such as DNA methylation, histone modifications, or small noncoding RNAs that are transmitted by the paternal germline at fertilization (Bohacek & Mansuy, 2015). Thus, studying animal models of experience-driven epigenetic inheritance may have major implications for elucidating novel mechanisms that contribute to the heritability of a wide range of human health conditions such as AUD.
Indeed, several studies have demonstrated that paternal preconception exposure to alcohol leads to developmental and behavioral alterations in offspring (see Finegersh et al., 2015 for review). Expanding on this literature, we previously investigated if paternal ethanol exposure impacts ethanol preference and sensitivity. We found that adult male mice exposed to chronic intermittent vapor ethanol (CIE) prior to mating with ethanol-naïve females produced male offspring with attenuated ethanol drinking behavior, increased sensitivity to the anxiolytic effects of ethanol, and increased brain-derived neurotropic factor (BDNF) gene expression in the ventral tegmental area (VTA) (Finegersh & Homanics, 2014). Furthermore, we found that paternal CIE (E)-sired males exhibit blunted acute and chronic stress-related phenotypes (Rompala, Finegersh, & Homanics, 2016). These results have major implications for AUD heritability, as they show that chronic exposure to ethanol prior to conception directly influences the inheritance of ethanol- and stress-related behaviors in offspring.
While these effects of paternal CIE are consistent with the surge of recent findings that show paternal experience can shape the behavioral phenotype of offspring, whether such intergenerational phenotypes are reproducible and sustained across various mouse strains is largely unknown. Indeed, changes in breeding strategy, such as the use of intercrossing vs outcrossing, have been shown to greatly affect the penetrance of paramutations in rodents (Yuan, Oliver, Schuster, Zheng, & Yan, 2015). As humans have a diverse genetic make-up, establishing whether models of intergenerational epigenetic inheritance are maintained across various rodent strains will be important for determining the translational implications of such findings.
Our previous two studies utilized CIE-exposed C57BL/6J (B6) male sires mated with Strain 129S1/SvImJ females to produce hybrid F1 male offspring that exhibited altered ethanol- and stress-related behaviors (Finegersh & Homanics, 2014; Rompala et al., 2016). In the current study, we tested the hypothesis that the effects of paternal CIE on ethanol- and stress-related behaviors would generalize to male offspring on an inbred genetic background. To this end, B6 males were exposed to CIE and mated with ethanol naïve B6 females to produce genetically identical F1 offspring for assessment of ethanol drinking behavior, sensitivity to acute ethanol injection and acute HPA axis responsivity. Our results confirm that paternal CIE produces decreased ethanol drinking behavior and increased ethanol sensitivity in inbred males of the next generation. Conversely, we did not see an effect of paternal CIE on HPA axis responsivity in male offspring. These results indicate that many, but not all effects of paternal preconception ethanol exposure are reproducible on inbred B6 animals.
Materials and Methods
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Eight-week-old, ethanol-naïve, specific pathogen free B6 and Strain 129S1/SvImJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Unless otherwise specified, mice were group-housed in individually ventilated microisolater cages under 12 hour light/dark cycles and had ad libitum access to food and water.
Chronic intermittent vapor ethanol exposure (CIE)
The CIE paradigm used to model paternal preconception ethanol exposure was modified only slightly from previously published methods (Finegersh & Homanics, 2014; Rompala et al., 2016). Briefly, group housed eight-week-old, B6 male mice were exposed to vapor ethanol (E) or room air control conditions (C) for 8 hours/day (0900 to 1700), 5 days/week (M–F) for six weeks. Sires were weighted weekly and blood ethanol concentrations (BECs) were measured following the final exposure of each week. Following the fifth week of exposure, mice were mated with one eight-week-old Strain 129S1/SvImJ females for two nights for the purpose of eliminating mature sperm that were not exposed to ethanol during all stages of spermatogenesis.
Immediately after the final ethanol exposure, E- and C-exposed males were bred in the home cage of two eight-week-old ethanol-naïve female B6 mice for 48 hours to produce F1 male and female offspring. Each C-sired and E-sired mouse was only used for one experiment (i.e. there was no repeated testing of any one mouse). For all experiments, no more than two mice of the same sex were used per litter. Offspring body weight measurements in Fig. 2 were only recorded from mice used in the two bottle free choice and acute ethanol injection experiments.
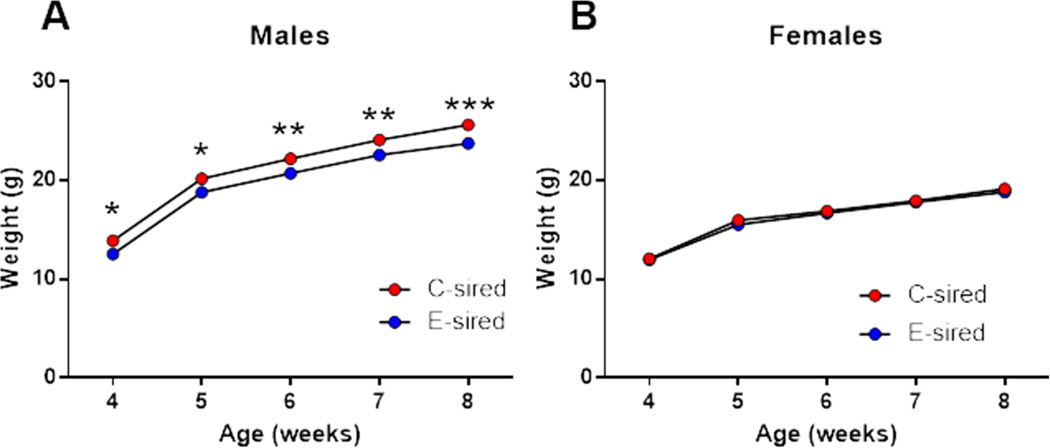
Figure 2. Paternal CIE reduces body weight selectively in male offspring.
(A) B6 E-sired males (n=19) showed decreased body weight vs C-sired males (n=15) at ages 4–8 weeks. (B) B6 E-sired females (n=23) showed no significant difference in body weight vs C-sired females (n= 24). Data presented as mean ± SEM. *=p<0.05, **=p<0.01, and ***=p<0.001.
Two bottle free choice ethanol drinking
Two bottle, free choice ethanol drinking behavior was performed as previously described (Finegersh & Homanics, 2014). Briefly, at 8 weeks age, ethanol-naïve male and female E- and C-sired B6 offspring were single housed and habituated to two ballbearing sipper-fitted 25 ml falcon tubes filled with water for one week. Following habituation, one tube was filled with ethanol at escalating concentrations of 3, 6, 9, 12, 15%, with each concentration tested for four days. Tube position and cages were changed every four days. Male mice used in the two bottle free choice drinking experiments were derived from 6 E-sired litters and 8 C-sired litters. Female mice were derived from 8 E-sired litters and 8 C-sired litters.
Two-Bottle Free Choice Saccharine and Quinine Drinking
Following testing at 15% ethanol, and a one-week washout period where only water was available, mice were tested for saccharin and quinine preference at two concentrations each with a one-week washout between tastants to control for sweet and bitter taste preferences, respectively.
Acute ethanol injection and successive elevated plus maze, open field, and accelerating rotarod tests
Eight-week-old male and female E- and C-sired B6 offspring were tested on the elevated plus maze, open field, and accelerating rotarod tasks all on the same day and in succession 10, 20, and 35 min, respectively, following an acute intraperitoneal injection of 0.9% saline (0.02 ml/g body weight) or 1.00 g/kg ethanol (0.02 ml/g of 5% ethanol in saline) as previously described (Finegersh & Homanics, 2014). Male mice used were derived from 10 E-sired litters and 7 C-sired litters for both saline and ethanol treatments. Female mice used in this experiment were derived from 6 E-sired litters and 5 C-sired litters for both saline and ethanol treatments.
Brain tissue processing and reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR)
Tissue was collected from eight-week old behaviorally-naïve E- and C-sired male mice. Mice were sacrificed between 1200 and 1600 hr during the light cycle. Brains were dissected and frozen with dried ice before being sectioned with a Microm HM 550 cryostat (Thermo Scientific, Waltham, MA). Using a 1 mm diameter micropuncher, three 300 micron thick tissue punches were collected from the VTA (approximately −5.2 to −6.1 mm, relative to bregma) (Paxinos & Franklin, 2001) into Trizol (Ambion, Grand Island, NY). Tissue was then lysed with a dounce homogenizer for RNA extraction using phenol-chloroform separation. Samples were further processed for RT-qPCR by DNAse I (Ambion) treatment, followed by final purification with RNA Clean and Concentrator (Zymo Research, Irvine, CA) and elution into 14 µl nuclease free water. Reverse transcription of RNA was performed with the iScript cDNA Synthesis Kit (BioRad Laboratories, Hercules, CA) according to manufacturer’s protocol. The cDNA product was diluted 1:10 before qPCR using BioRad SYBR Green Fluorescent Master Mix and a BioRad iCycler. Oligo sequences were: brain derived neurotropic factor (BDNF) exon IX Forward (F): 5’-AGC CTC CTC TAC TCT TTC TGC TG-3’ and BDNF exon IX reverse (R): 5’-GTG CCT TTT GTC TAT GCC CCT G; β-actin F: 5’-CGT TGA CAT CCG TAA AGA CC-3’ and R: 5’-AAC AGT CCG CCT AGA AGC AC-3’. Threshold cycle values for each gene were normalized within sample to β-actin and then between groups for computation of delta delta cycle threshold (ΔΔCt) to calculate fold change in mRNA expression. Due to limited animal availability, we restricted this experiment to male offspring. E- and C-sired males used in this experiment were derived from 4 E-sired litters and 4 C-sired litters.
Acute restraint stress and measurement of plasma corticosterone (CORT)
Eight-week-old male E- and C-sired offspring were subjected to a 15 min restraint stress exposure. All animals were tested between three and five hours after lights on (1000–1200 hr). Briefly, mice were restrained in conical plastic tubes with several air hole perforations near the animal’s head and an opening for the tail. After the 15 minute restraint, each mouse was returned to its home cage. Tail blood (<10ul) was collected with heparin-coated capillary tubes (Drummond, Broomall, PA) at time points 0, 15, 30, and 90 minutes from the onset of restraint. Blood samples were centrifuged for 10 minutes at 4500 × g to separate plasma for measurement of CORT with an enzyme immunoassay (Enzo Life Sciences, Farmingdale, NY). Samples were diluted 1:40 and run in duplicate. The correlation coefficient for duplicate measures in our assay was r= 0.99. The reported sensitivity of this kit for detecting CORT concentrations ranges from 0.032–20 ng/ml. Due to limited animal available, we restricted this experiment to male offspring. Male mice used in this experiment were derived from 8 E-sired litters and 9 C-sired litters.
Statistical Analysis
Behavioral and HPA axis responsivity experiments were analyzed using two way ANOVAs with or without repeated measures where appropriate. For ANOVA results reaching statistical significance (p<0.05), post-hoc pairwise comparisons were made using Fisher’s LSD test. For rt-qPCR results, analysis was performed using Student’s t-test.
Results
Paternal preconception CIE exposure
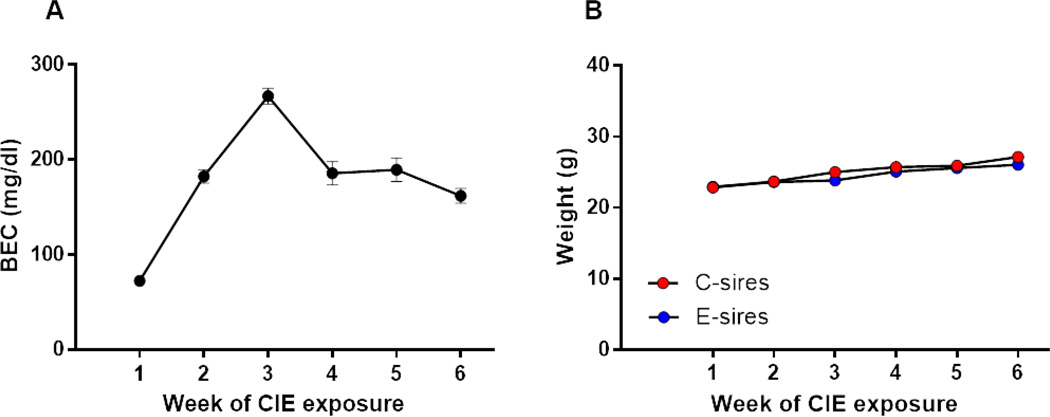
B6 males were exposed to CIE or room air conditions for six weeks. The average BEC (Fig. 1A) across all weeks of paternal CIE was 180.2 ± 14.7 mg/dl (mean ± S.E.M.). There was a significant effect of time on sire body weight (F(5,110) = 92.6, p <0.001; Fig. 1B) but no effect of ethanol exposure or ethanol exposure × time interaction. These results indicate that animals gained weight and there was no difference in body weight between E-sires and C-sires over the course of the exposure period.
Figure 1. Paternal preconception CIE exposure.
(A) Mean blood ethanol concentrations (BEC) are shown for E-sires. (B) There was no effect of CIE on the body weights of E-sires vs C-sires (n=12, 12; E-sires, C-sires). Data presented as mean ± SEM.
Paternal CIE reduces post-weaning body weight selectively in B6 male offspring
There was a significant effect of age (F(4,128)=1177, p<0.001) and sire (F(1,32)=9.93; p<0.01; Fig. 2A) on male offspring body weight indicating that E-sired males weighted significantly less vs C-sired males. Fisher’s LSD post-hoc test revealed that E-sired males had significantly decreased body weight at postnatal weeks 4–5 (p<0.05), 6–7 (p<0.01) and 8 (p<0.001) vs C-sired males. Analysis of female offspring body weights revealed a significant effect for age (F(4,180)=650.7, p<0.0001; Fig. 2B), but no effect of sire and no age × sire interaction.
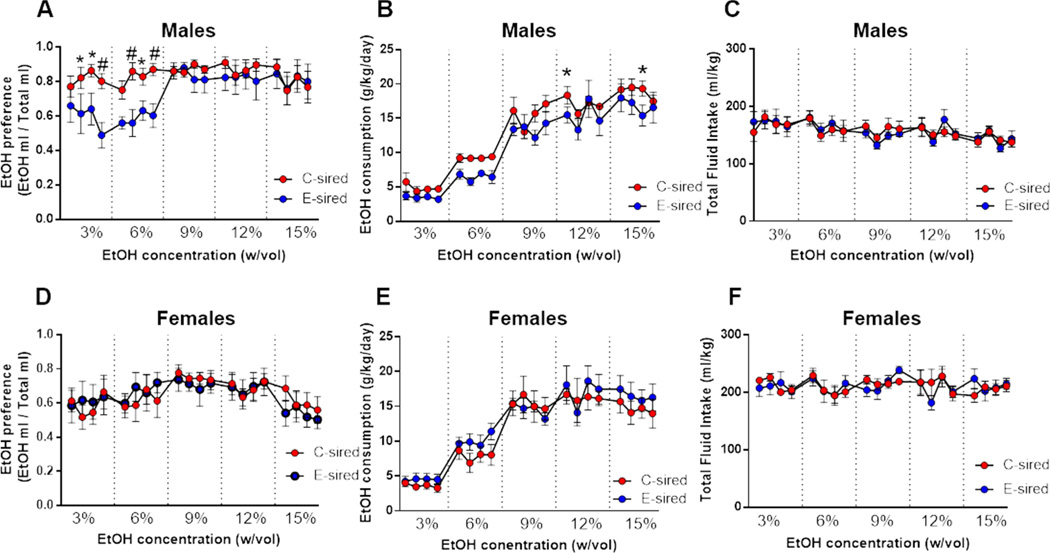
Paternal CIE reduces ethanol drinking behaviors selectively in male offspring
E- and C-sired adult male and female offspring were tested for ethanol drinking behavior in a two bottle choice test at sequential ethanol concentrations of 3, 6, 9, 12, and 15 % (w/vol) for 4 days each. Analysis of ethanol preference in males revealed a significant effect of ethanol concentration (F(19,266)=3.165, p<0.001) with no effect of sire, and a significant sire × ethanol concentration interaction (F(19,266)= 1.8, p<0.05; Fig. 3A); Fisher’s LSD post-hoc analysis revealed significantly reduced ethanol preference during three days each at the 3 and 6% ethanol concentrations (p<0.05 and 0.01). In addition, there was a significant effect for ethanol concentration (F(19, 266)=46.57, p<0.001) and sire (F(1,14) =5.8, p<0.05; Fig. 3B) with no ethanol concentration × sire interaction for ethanol consumption. Fisher’s LSD post-hoc analysis of ethanol consumption over individual days revealed significantly reduced ethanol consumption by E-sired vs C-sired males on a single day each of 9% and 15% ethanol concentrations (p<0.05). There was no effect of ethanol concentration, sire, or ethanol concentration × sire interaction on total fluid intake (Fig. 3C).
Figure 3. Paternal CIE attenuates ethanol drinking behavior selectively in male offspring.
(A) E-sired males (n=8) showed reduced ethanol preference and (B) ethanol consumption over multiple days of the two bottle choice test vs C-sired males (n=8). (C) There was no difference in total fluid intake for E-sired vs C-sired males. (D) E-sired (n=8) and C-sired (n=8) females showed no significant difference in ethanol drinking preference, (E) ethanol consumption, or (F) total fluid intake. Data presented as mean ± SEM. *=p<0.05. #=p<0.01.
In contrast to males, female offspring showed no effect of ethanol concentration, sire, or ethanol concentration × sire interaction on ethanol preference (Fig. 3D). For ethanol consumption, there was a significant effect of ethanol concentration (F(19,266)=26.84, p<0.001) with consumption increasing at higher concentrations, but no effect of sire or ethanol concentration × sire interaction (Fig. 3E). Finally, there was no effect of ethanol concentration, sire, or ethanol concentration × sire interaction on total fluid intake (Fig. 3F).
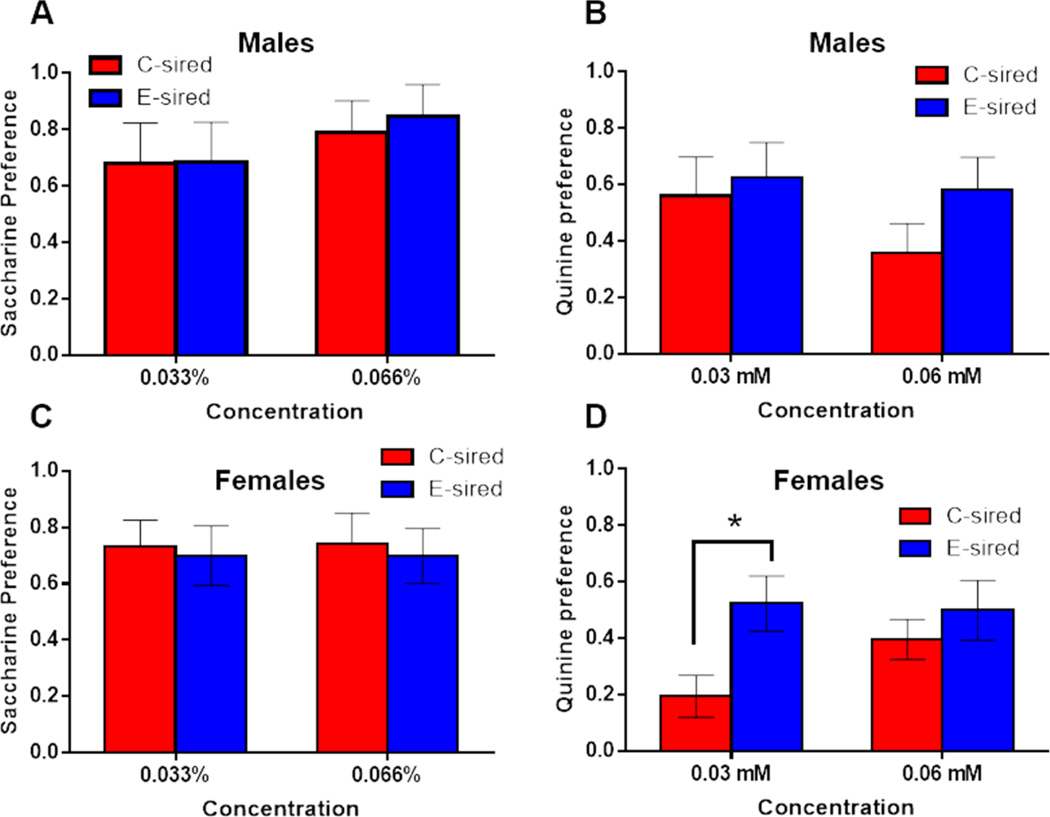
Paternal CIE does not affect offspring drinking preference for sweet and bitter solutions
Following a one-week washout period, male and female offspring were tested in a two bottle choice paradigm with saccharine or quinine, controlling for preference of sweet and bitter tastants, respectively. In males, there was no effect of tastant concentration, sire, or their interaction on saccharin or quinine preference (Figs. 4A–B). In female offspring, no effect of concentration, sire, or their interaction was observed for saccharin preference (Fig. 4C). For quinine preference, there was no effect for concentration or sire, but a significant concentration × sire interaction (F(1, 14)=7.17, p<0.05; Fig. 4D); Fisher’s LSD post-hoc test revealed increased quinine preference at the 0.033 mM concentration for E-sired vs C-sired females (p<0.05).
Figure 4. Effects of paternal CIE on offspring saccharin and quinine preference.
In control tests for (A) saccharin and (B) quinine drinking preference, there was no significant difference for E-sired (n=8) vs C-sired males (n=8). In females, (C) saccharin preference was not different for E-sired (n=8) vs C-sired (n=8) groups, but E-sired females did have greater (D) quinine preference specifically at the 0.03 mM concentration. Data presented as mean ± SEM. *=p<0.05.
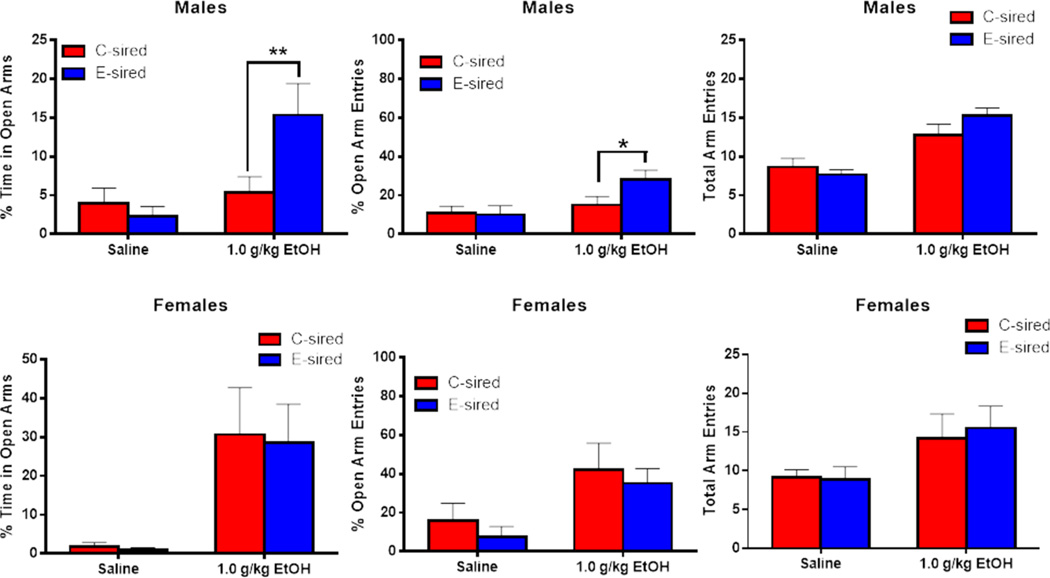
Paternal CIE increased sensitivity to the anxiolytic effects of ethanol selectively in male offspring
Both male and female offspring were assessed for sensitivity to an acute low dose of ethanol (1.0 g/kg) or saline in a sequential three test behavioral battery (elevated plus maze, open field, rotarod). Ten minutes following ethanol or saline injections, mice were assessed for exploratory behavior of open and closed arms on the elevated plus maze. For male offspring, there was a significant effect of treatment (F(1, 40) = 8.226, p<0.01) and a treatment × sire interaction (F(1,40) =5.358, p<0.05) on percent time spent in the open arms (Fig. 5A); Fisher’s LSD post-hoc analysis revealed that E-sired males showed a significant increase in open arm time vs saline-injected E-sired males (p<0.001) and vs ethanol-injected C-sired males (p<0.01). For open arm entries (Fig. 5B), there was an increase with ethanol treatment (F(1, 41) = 6.53, p<0.05) but no effect of sire or treatment × sire interaction; Fisher’s LSD post hoc tests revealed significantly increased open arm entries in E-sired vs C-sired males after ethanol injection (p<0.05). For total arm entries, there was a significant increase with ethanol treatment (F(1,41)=37.74, p<0.001, Fig. 5C), but no significant effect for sire or sire × treatment interaction.
Figure 5. Paternal CIE increases sensitivity to acute ethanol selectively in male offspring.
(A) In the elevated plus maze, following an acute injection with 1 g/kg ethanol, E-sired males (n=11) spent more time in the open arm (% of total time) and made more (B) open arm entries (% of total entries) vs C-sired males (n=10). (C) ethanol injection increased total arm entries, but there was no difference between E-and C-sired males. There was no difference between E-sired and C-sired males for all elevated plus maze measures following saline injection (n=12 for E-sired; n=11 for C-sired). (D) There was no difference between E-sired (n=6) and C-sired (n=6) female mice for time in the open arm, (E) open arm entries, or (F) total arm entries following acute ethanol injection. Saline-treated E-sired and C-sired females (n=7 for E-sired; n=7 for C-sired) did not differ across all measures in the elevated plus maze. Data presented as mean ± SEM. *=p<0.05, **=p<0.01, ***=p<0.001.
Testing of female offspring on the elevated plus maze revealed a significant increase in open arm time following ethanol treatment (F(1, 22) = 15.11; p<0.001, Fig. 5D), but no effect of sire or sire × treatment interaction. For open arm entries, there was a significant increase with ethanol treatment (F(1, 22) = 8.805, p<0.01, Fig. 5E), but no effect of sire and no treatment × sire interaction. For total arm entries, there was a significant increase with ethanol treatment (F(1,22)=6.832, p<0.05; Fig. 5F), but no effect for sire or sire × treatment interaction.
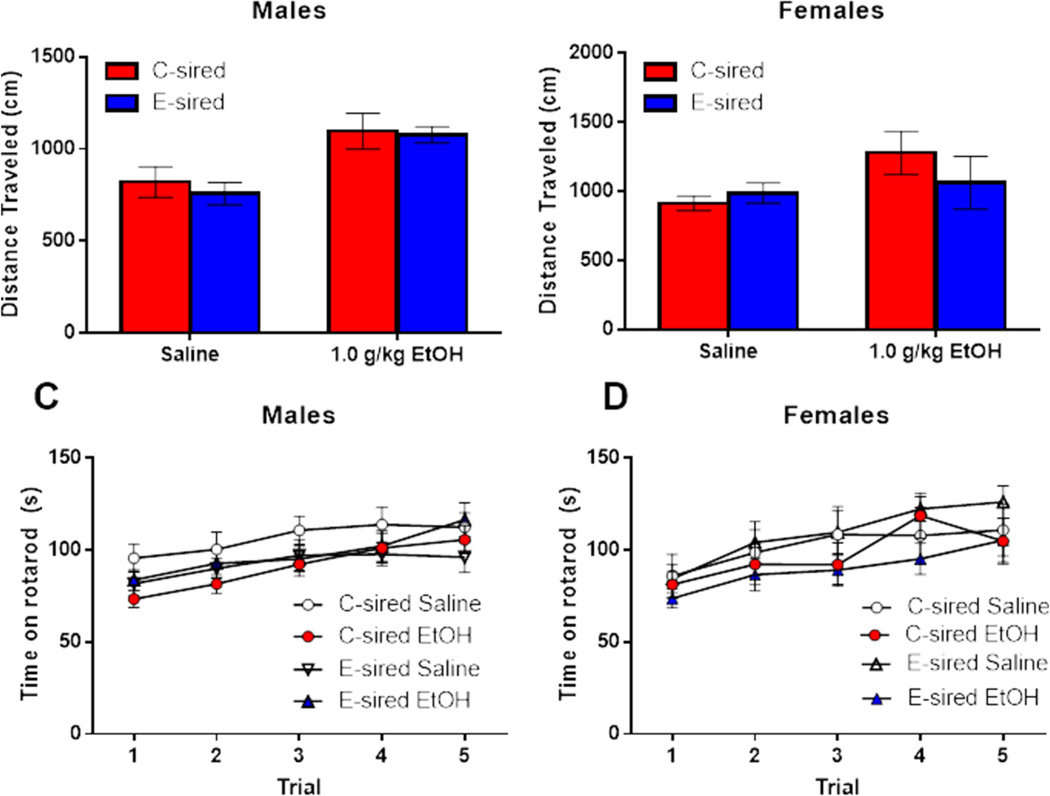
Five minutes after the elevated plus maze (i.e., 20 min following ethanol or saline injection), mice were examined during a 10 min open field test for ethanol-induced locomotor activity. For males, there was a significant increase in distance traveled in the open field test following ethanol treatment (F(1,40)=16.84, p<0.001; Fig. 6A), but no effect of sire and no treatment × sire interaction. For females, there was no effect of treatment, sire, or treatment × sire interaction (Fig. 6B).
Figure 6. No effects of paternal CIE on exploratory behavior or accelerating rotarod performance after acute ethanol injection.
(A) E-sired (ethanol, n=11; saline, n=12) and C-sired (ethanol, n=10; saline, n=11) male mice performed similarly in the open field test following an acute ethanol injection. In the accelerating rotarod task, (B) E-sired (ethanol, n=5; saline, n=7) and C-sired (ethanol, n=6; saline, n=7) females responded similarly to acute ethanol in the open field test. (C) C-sired males (ethanol, n=10; saline, n=11) and E-sired males (ethanol, n=11; saline, n=12) did not show a significant effect for ethanol injection on rotarod performance. (D) There was no effect of ethanol on rotarod performance in C-sired females (ethanol, n=6; saline, n=7) or in E-sired females (ethanol, n=6; saline, n=7). Data presented as mean ± SEM. ***=p<0.001.
Five minutes following the open field test (i.e., 35 min following ethanol or saline injection), mice underwent five trials on an accelerating rotarod test to assess basal motor coordination and ethanol-induced ataxia. Males showed a significant improvement in time spent on the rotarod over the five trials (F(4,156)=10.83, p<0.001; Fig. 6C), but no effect of treatment or trial × treatment interaction. Similarly, female mice showed a significant effect for trial (F(4,88)=9.04, p<0.001; Fig. 6D), with no effect for treatment or interaction.
Paternal CIE increases BDNF gene expression in the VTA of male offspring
Comparing adult E- and C-sired male offspring, we found that BDNF gene expression was increased in the VTA of E-sired vs C-sired males (t(6)= 2.94, p<0.05; Fig. 7A). In addition, when comparing E- and C-sired males for acute CORT responses to 15 min restraint stress, there was an effect for time of measurement, reflected by a sharp increase in corticosterone levels at 15 and 30 minutes from the onset of restraint stress (F(3,45)=110.2, p<0.001; Fig. 7B); however, there was no effect for sire and no sire × time interaction.
Figure 7. Paternal CIE increases BDNF mRNA expression VTA of male offspring.
(A) There was a significant increase in BDNF mRNA expression in the VTA of E-sired males vs C-sired males (n= 4/4, C-sired, E-Sired). (B) No difference in corticosterone levels was observed between groups following 15 minutes of acute restraint stress (shaded bar) (n= 9/8, C-sired, E-sired). Data presented as mean ± SEM. *=p<0.05.
Discussion
Here, we report that paternal preconception chronic ethanol exposure imparts decreased ethanol drinking preference at low concentrations, increased sensitivity to the anxiolytic effects of ethanol, and increased BDNF gene expression in the VTA to adult male offspring. Whereas our prior study illustrated that E-sired F1 offspring on a Strain 129S1/SvImJ × B6 hybrid genetic background exhibited altered ethanol-related behaviors (Finegersh & Homanics, 2014), the current study extends those observations and demonstrates the same intergenerational phenotype using genetically identical, inbred B6 animals. Furthermore, the male offspring-specific intergenerational effects of paternal CIE are also consistent with our prior two studies (see Finegersh & Homanics 2014 or Rompala et al. 2016 for discussion of sex-specific effects of paternal CIE). There is an increasing emphasis from the National Institutes of Health for investigators to demonstrate that significant findings are in fact reproducible. Moreover, there is some concern that high profile epigenetic inheritance studies are vulnerable to reporting results with inflated statistical significance (Francis, 2014). Therefore, our results show that intergenerational effects of paternal CIE on ethanol-related behaviors in male offspring are in fact reproducible and also observable on an inbred genetic background.
The consistent results between this study and Finegersh & Homanics, 2014 are remarkable considering the various dissimilarities between the two maternal mouse strains used. Strain 129 and B6 mice differ on measures of stress reactivity (van Bogaert et al., 2006), taste perception (Bachmanov, Tordoff, & Beauchamp, 1996), and two bottle choice ethanol drinking (Rhodes et al., 2007). Moreover, Strain 129 and B6 dams provide different levels of maternal care (Gabriel & Cunningham, 2008) and each strain differentially regulates the maintenance of paternally inherited methylation at intracisternal A particles in utero (Rakyan et al., 2003). Therefore, despite the heterogeneous behavioral and epigenetic profile of these two mouse strains, our findings demonstrate a reproducible effect for paternal CIE on ethanol-related behaviors in male offspring.
While our finding that paternal CIE confers an attenuated ethanol drinking phenotype to male offspring was originally unanticipated given the tendency for alcoholism to run in families, the outcome is similar to other recent paternal preconception studies in rodents. For instance, male mice exposed to chronic cocaine or stress were found to sire offspring with decreased cocaine preference and blunted stress responsivity, respectively (Rodgers et al., 2013; Vassoler et al., 2013). Therefore, it is possible that paternal CIE promotes the inheritance of reduced ethanol preference, protecting male offspring against excessive ethanol consumption.
While we have replicated our previous finding that paternal CIE reduces ethanol drinking preference selectively in male offspring, it is worth noting certain limitations. For instance, both the current study and our results from Finegersh & Homanics, 2014 only report effects of paternal CIE on ethanol drinking preference in male offspring at the lower ethanol concentrations tested (primarily 3 and 6 percent). It is unclear whether this effect is specific to low concentrations of ethanol or the sequence of concentrations tested (ascending from 3 to 15 percent). In addition, although we have observed sex-specific effects of paternal CIE, it is conceivable that effects on female offspring are confounded or masked by factors such as estrus cycle (Meziane, Ouagazzal, Aubert, Wietrzych, & Krezel, 2007) or altered tastant sensitivity as reflected by our present finding of reduced quinine preference in E-sired females (Fig. 4). Thus, to further define the ethanol drinking phenotype in E-sired offspring, additional experiments and drinking paradigms need to be considered (e.g. drinking in the dark, operant self-administration). Likewise, alternative paternal preconception ethanol exposure models may be necessary as it is unknown whether paternal voluntary ethanol consumption would have the same intergenerational effects as our forced vapor exposure that produces higher BECs and HPA stress axis activation (Rivier, 2014).
In addition to decreased ethanol drinking preference, and again consistent with our original findings from Finegersh & Homanics, 2014, we found that E-sired male offspring exhibited heightened sensitivity to an anxiolytic dose of ethanol in the elevated plus maze. Decreased subjective response to ethanol is associated with increased risk for AUD (Schuckit, 1985; Schuckit & Smith, 1996), suggesting that ethanol sensitivity is inversely associated with AUD. This would suggest that increased ethanol sensitivity in E-sired males is consistent with the reduced ethanol drinking behavior phenotype. However, we did not find an effect for paternal CIE on basal or ethanol-induced locomotor activity and motor coordination. Hence, only a subset of ethanol-induced behavioral measures are impacted in E-sired male offspring. One notable limitation to these experiments is the timing of the behavioral battery and fixed sequence of experiments. The elevated plus maze was conducted 10 min after ethanol or saline injection and then sequentially followed by the open field test (20 min post injection) and accelerating rotarod (35 min post injection). As a result, we cannot rule out that the intergenerational effect of paternal CIE exclusively in the elevated plus maze may have been due to the timing and/or sequence of experimentation.
In addition to altered intergenerational ethanol-related behaviors, there was a significant increase in BDNF gene expression in the VTA of E-sired males, in accordance with our previous findings (Finegersh & Homanics, 2014). Expression of BDNF in various brain regions has been found to mediate alcohol drinking behavior in rodents (Pandey, 2016). Indeed, innate BDNF expression is increased in the VTA of alcohol-avoiding rats (Raivio, Miettinen, & Kiianmaa, 2014). Infusion of BDNF into the VTA is sufficient to shift conditioned place preference for alcohol from a dopamine-dependent to dopamine-independent behavior (Ting et al., 2013). Thus, mechanisms governing ethanol motivation may differ between ethanol-sired and control male mice and BDNF may be an attractive target for further elucidating the neurobiological substrates involved.
Lastly, we did find that some of the effects of paternal CIE on male offspring varied with maternal strain. First, contrary to our findings in Finegersh & Homanics 2014 that paternal CIE increases postweaning body weight in hybrid male offspring, here, there was a small, but significant reduction of postweaning body weight in E-sired male offspring on an inbred background. However, this outcome is not surprising given that many paternal ethanol exposure studies have found either increased or decreased offspring body weight using different rodent strains or exposure paradigms (Knezovich & Ramsay, 2012; Ledig et al., 1998; Mankes et al., 1982). The second inconsistency between studies is the absence of an effect of paternal CIE on acute HPA axis responsivity in F1 inbred male offspring, counter to our published findings showing that paternal CIE produced stress hyporesponsivity phenotypes in F1 hybrid male offspring (Rompala et al., 2016). Indeed, the different outcome may be due to aforementioned differences between B6 and Strain 129 dams. For instance, variations in maternal behavior, as seen with B6 and Strain 129 dams, has a significant effect on adult HPA axis responsivity in adult offspring (Caldji, Diorio, & Meaney, 2000). Thus, additional experiments, such as employing a cross-fostering strategy, may be necessary to determine whether the strain-dependent effect of paternal CIE on body weight and intergenerational HPA axis responsivity is explained by differences in maternal biology or maternal care.
Between this study and the results from Finegersh & Homanics, 2014, we have established a reproducible model of paternal preconception ethanol exposure that stably impacts ethanol drinking behavior and ethanol sensitivity selectively in male offspring. This model will facilitate future experiments attempting to identify the causal mechanisms in sperm that drive heritable changes in complex ethanol-related behaviors. Along with the paternal genome, sperm transmit epigenetic mechanisms including but not limited to DNA methylation, histone modifications, and small noncoding RNAs to the oocyte at fertilization (Schagdarsurengin & Steger, 2016) (Rando, 2016). Small noncoding RNAs are an attractive mechanism for epigenetic inheritance as recent studies have shown they can be transmitted from the somatic cells of the central nervous system to the germline (Devanapally, Ravikumar, & Jose, 2015), possibly through exosome signaling (Cossetti et al., 2014; Sharma et al., 2016). Moreover, paternal experience such as chronic stress alters sperm miRNA expression (Gapp et al., 2014; Rodgers et al., 2013) and injection of the most altered miRNAs into fertilized oocytes from normal donor mice can recapitulate the intergenerational effects of paternal stress in adult progeny (Rodgers, Morgan, Leu, & Bale, 2015). In addition, other studies have shown that paternal experience alters DNA methylation (Dias & Ressler, 2014; Finegersh & Homanics, 2014; Govorko, Bekdash, Zhang, & Sarkar, 2012) and histone modifications (Siklenka et al., 2015; Vassoler et al., 2013) in sperm although technical limitations have complicated identifying a causal role for these mechanisms in epigenetic inheritance of paternal experience. The germline function of such mechanisms will likely become more delineated with the advancement of novel technologies for targeted chromatin remodeling such as zinc-finger protein or CRISPR/Cas systems (Thakore, Black, Hilton, & Gersbach, 2016).
In summary, paternal preconception ethanol exposure confers reduced ethanol drinking behavior, increased sensitivity to ethanol, and increased BDNF gene expression in the VTA to male offspring. The evidence for these intergenerational phenotypes is robust as we have now observed effects of paternal CIE on both hybrid and inbred male offspring. Identifying heritable epigenetic mechanisms that confer resistance to excessive ethanol drinking behavior has major implications for the development of novel AUD prevention and treatment strategies. Therefore, future studies will aim to identify sperm-borne epigenetic mechanisms with a causal role in intergenerational ethanol-related behaviors.
Highlights.
Paternal preconception ethanol exposure reduced ethanol drinking behavior and increased ethanol sensitivity selectively in male offspring
Paternal ethanol preconception exposure increased BDNF in the VTA of male offspring
The effects of paternal preconception ethanol exposure on intergenerational ethanol-related behaviors are observable in inbred and hybrid male offspring
Acknowledgments
We would like to thank Carolyn Ferguson for providing expert technical assistance. This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism awarded to GRR (F31AA024670), AF (F30AA021632) and GEH (R37AA010422),
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res. 1996;20(2):201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Mansuy IM. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat Rev Genet. 2015;16(11):641–652. doi: 10.1038/nrg3964. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48(12):1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351(6271):397–400. doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]
- Cossetti C, Lugini L, Astrologo L, Saggio I, Fais S, Spadafora C. Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: possible transport by exosomes. PLoS One. 2014;9(7):e101629. doi: 10.1371/journal.pone.0101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanapally S, Ravikumar S, Jose AM. Double-stranded RNA made in C. elegans neurons can enter the germline and cause transgenerational gene silencing. Proc Natl Acad Sci U S A. 2015;112(7):2133–2138. doi: 10.1073/pnas.1423333112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 2014;17(1):89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegersh Homanics GE. Paternal alcohol exposure reduces alcohol drinking and increases behavioral sensitivity to alcohol selectively in male offspring. PLoS One. 2014;9(6):e99078. doi: 10.1371/journal.pone.0099078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegersh A, Rompala GR, Martin DI, Homanics GE. Drinking beyond a lifetime: New and emerging insights into paternal alcohol exposure on subsequent generations. Alcohol. 2015;49(5):461–470. doi: 10.1016/j.alcohol.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G. Too much success for recent groundbreaking epigenetic experiments. Genetics. 2014;198(2):449–451. doi: 10.1534/genetics.114.163998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel KI, Cunningham CL. Effects of maternal strain on ethanol responses in reciprocal F1 C57BL/6J and DBA/2J hybrid mice. Genes Brain Behav. 2008;7(3):276–287. doi: 10.1111/j.1601-183X.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014;17(5):667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorko D, Bekdash RA, Zhang C, Sarkar DK. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biol Psychiatry. 2012;72(5):378–388. doi: 10.1016/j.biopsych.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezovich JG, Ramsay M. The effect of preconception paternal alcohol exposure on epigenetic remodeling of the h19 and rasgrf1 imprinting control regions in mouse offspring. Front Genet. 2012;3:10. doi: 10.3389/fgene.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledig M, Misslin R, Vogel E, Holownia A, Copin JC, Tholey G. Paternal alcohol exposure: developmental and behavioral effects on the offspring of rats. Neuropharmacology. 1998;37(1):57–66. doi: 10.1016/s0028-3908(97)00185-8. [DOI] [PubMed] [Google Scholar]
- Mankes RF, LeFevre R, Benitz KF, Rosenblum I, Bates H, Walker AI, et al. Paternal effects of ethanol in the long-evans rat. J Toxicol Environ Health. 1982;10(6):871–878. doi: 10.1080/15287398209530302. [DOI] [PubMed] [Google Scholar]
- Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 2007;6(2):192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Pandey SC. A Critical Role of Brain-Derived Neurotrophic Factor in Alcohol Consumption. Biol Psychiatry. 2016;79(6):427–429. doi: 10.1016/j.biopsych.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates (Vol. Second Edition) Academic Press; 2001. [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. American Journal of Psychiatry. 1999;156(1):34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Raivio N, Miettinen P, Kiianmaa K. Innate BDNF expression is associated with ethanol intake in alcohol-preferring AA and alcohol-avoiding ANA rats. Brain Res. 2014;1579:74–83. doi: 10.1016/j.brainres.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Rakyan VK, Chong S, Champ ME, Cuthbert PC, Morgan HD, Luu KV, et al. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc Natl Acad Sci U S A. 2003;100(5):2538–2543. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando OJ. Intergenerational Transfer of Epigenetic Information in Sperm. Cold Spring Harb Perspect Med. 2016;6(5) doi: 10.1101/cshperspect.a022988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6(1):1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Role of hypothalamic corticotropin-releasing factor in mediating alcohol-induced activation of the rat hypothalamic-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35(2):221–233. doi: 10.1016/j.yfrne.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33(21):9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A. 2015;112(44):13699–13704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Finegersh A, Homanics GE. Paternal preconception ethanol exposure blunts hypothalamic-pituitary-adrenal axis responsivity and stress-induced excessive fluid intake in male mice. Alcohol. 2016;53:19–25. doi: 10.1016/j.alcohol.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagdarsurengin U, Steger K. Epigenetics in male reproduction: effect of paternal diet on sperm quality and offspring health. Nat Rev Urol. 2016 doi: 10.1038/nrurol.2016.157. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Ethanol-Induced Changes in Body Sway in Men at High Alcoholism Risk. Archives of General Psychiatry. 1985;42(4):375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Archives of General Psychiatry. 1996;53(3):202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351(6271):391–396. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siklenka K, Erkek S, Godmann M, Lambrot R, McGraw S, Lafleur C, et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science. 2015;350(6261):aab2006. doi: 10.1126/science.aab2006. [DOI] [PubMed] [Google Scholar]
- Thakore PI, Black JB, Hilton IB, Gersbach CA. Editing the epigenome: technologies for programmable transcription and epigenetic modulation. Nat Methods. 2016;13(2):127–137. doi: 10.1038/nmeth.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting AKR, Vargas-Perez H, Bufalino MR, Bahi A, Dreyer JL, Tyndale RF, et al. Infusion of brain-derived neurotrophic factor into the ventral tegmental area switches the substrates mediating ethanol motivation. Eur J Neurosci. 2013;37(6):996–1003. doi: 10.1111/ejn.12105. [DOI] [PubMed] [Google Scholar]
- Treutlein J, Rietschel M. Genome-wide association studies of alcohol dependence and substance use disorders. Curr Psychiatry Rep. 2011;13(2):147–155. doi: 10.1007/s11920-011-0176-4. [DOI] [PubMed] [Google Scholar]
- van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der Gugten J, Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006;5(2):139–149. doi: 10.1111/j.1601-183X.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013;16(1):42–47. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Enoch MA, Prescott CA. The influence of gene-environment interactions on alcohol consumption and alcohol use disorders: A comprehensive review. Clinical Psychology Review. 2011;31(5):800–816. doi: 10.1016/j.cpr.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystrom E, Reichborn-Kjennerud T, Aggen SH, Kendler KS. Alcohol Dependence in Men: Reliability and Heritability. Alcoholism-Clinical and Experimental Research. 2011;35(9):1716–1722. doi: 10.1111/j.1530-0277.2011.01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Oliver D, Schuster A, Zheng H, Yan W. Breeding scheme and maternal small RNAs affect the efficiency of transgenerational inheritance of a paramutation in mice. Sci Rep. 2015;5:9266. doi: 10.1038/srep09266. [DOI] [PMC free article] [PubMed] [Google Scholar]