Abstract
We retrospectively evaluated the effects of 6% hydroxyethyl starch (HES) 130/0.4 on postoperative blood loss and acute kidney injury (AKI) in patients undergoing off-pump coronary artery bypass grafting (OPCAB).
Electronic medical records of 771 patients who underwent OPCAB in our hospital between July 2012 and July 2014 were reviewed, and 249 patients without intraoperative HES-exposure (group NoHES) were matched 1:N with intraoperative HES-exposed 413 patients (group HES) based on propensity score. The effects of intraoperative HES on postoperative cumulative blood loss within the first 24 hours, need for bleeding-related reoperation, and occurrence of postoperative AKI (determined by KDIGO and RIFLE criteria) were analyzed.
In our propensity score matched cohort, there were no significant differences between groups for median postoperative 24 hours blood loss (525 mL in group HES vs. 540 mL in group NoHES, P = .203) or need for bleeding-related reoperation (OR, 2.44; 95% confidence interval [CI], 0.64–9.34, P = .19). However, postoperative AKI (assessed by 2 criteria) occurred more frequently in group HES than in group NoHES (by KDIGO criteria: 10.7% vs. 3.6%; OR 3.43 [95% CI, 1.67–7.04]; P < .001 and by RIFLE criteria: 9.6% vs. 2%; OR 3.32 [95% CI, 1.34–8.24]; P = .01). The median volume of infused HES per patient weight was 16 mL/kg in group HES.
In the patients undergoing OPCAB, intraoperative 6% HES 130/0.4 did not increase postoperative bleeding. However, renal safety remains a concern. Intraoperative use of HES should be determined cautiously during OPCAB.
Keywords: acute kidney injury, colloid, hydroxyethyl starch, off-pump coronary arterial bypass grafting, postoperative bleeding
1. Introduction
Since its development in the 1960s, intravenous hydroxyethyl starch (HES) solution has been popularly used as a volume expander in hypovolemic patients. However, due to concerns regarding increased risk of mortality and severe renal injury, in July 2013, the US Food and Drug Administration (FDA) recommended avoiding the use of HES in critically ill patients. Whether similar adverse effects occur in the surgical population has not been clarified,[1] and clinicians are advised to avoid HES for patients undergoing open heart surgery with cardiopulmonary bypass due to excess bleeding risk. However, detailed information on the use of different HES products or the use of HES during cardiac surgery without cardiopulmonary bypass is lacking.
The molecular weight and molar substitution of HES have been optimized during the last few decades, and modern starches show better outcomes, especially for coagulation, compared with older starches.[2–4] Recent meta-analyses evaluating the latest generation HES (130/0.4) in surgical patients reported no clear evidence that intraoperative HES increases the risk of adverse outcomes such as mortality or renal injury.[1,5–7] However, the number of clinical trials analyzed was limited, and the majority of included cardiac surgeries were open heart procedures with cardiopulmonary bypass which is known to be associated with hematologic responses and organ injuries.[8]
Because off-pump coronary arterial bypass (OPCAB) is a major cardiac surgery that may be accompanied by a large volume of intravenous fluid administration, evaluating the clinical effect of HES on postoperative bleeding and renal injury in this surgical population seems to be essential. Moreover, the effects of HES could be interpreted in OPCAB patients without the complex influences of cardiopulmonary bypass. However, there is insufficient data on the clinical effects of HES in OPCAB.[2,9,10]
Therefore, we evaluated the effects of balanced 6% HES 130/0.4 (Volulyte, Fresenius Kabi, Bad Homburg, Germany) on postoperative blood loss and kidney injury in a retrospective propensity score-matched cohort study of patients undergoing OPCAB.
2. Methods
2.1. Study design and patient population
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2014-08-058) and was conducted in accordance with the principles of the Declaration of Helsinki. As this was a retrospective study using electronic medical records, the requirement for individual informed consent was waived. The study population consisted of adult patients older than 20 years who underwent OPCAB procedures performed by a single surgeon (Y. T. Lee) between July 2012 and July 2014 at Samsung Medical Center. In June 2013, the US FDA and Health Canada issued a warning on the use of HES solutions; accordingly, the Korean FDA advised health professionals to comply with the same recommendations. Until then, we used 6% HES 130/0.4 in a balanced electrolyte solution (Volulyte, Fresenius Kabi, Bad Homburg, Germany) as volume-expanding fluid in patients undergoing cardiac surgery at our hospital, but have avoided the use of HES solutions during all cardiac surgeries since July 2013. Patients were excluded if they had undergone intraoperative cardiopulmonary bypass or perioperative extracorporeal membrane oxygenation (ECMO) therapy. For patients who underwent several surgeries, we included only the first surgery in the analysis.
2.2. Data collection
The electronic medical records of enrolled patients were reviewed, and pre-, intra-, and postoperative data were collected. Data describing postoperative chest tube drainage and laboratory data including blood hemoglobin, platelet, and serum creatinine levels were extracted automatically from the electronic medical records with the aid of the hospital's medical information department. Postoperative outcome data were collected by manual review of each case by 2 researchers (JJM and SJ) who were blinded to the use of HES solution.
2.3. Perioperative coagulation management
The perioperative coagulation management strategy was as follows: for all patients who took either aspirin or clopidogrel, the medication was continued until the day of the surgery and resumed as soon as possible after surgery. If the patients had received dual antiplatelet therapy with aspirin and clopidogrel, only aspirin was continued until the day of the surgery, while clopidogrel was discontinued 2 days before surgery. Intraoperatively, the patients were given an initial dose of heparin (1.5 mg/kg) and periodic supplemental doses to maintain an activated clotting time >350 seconds. To neutralize heparin at the end of the surgery, protamine was given in a 0.5:1 ratio to the dose of heparin. If the follow-up activated clotting time was longer than 140 seconds, an additional dose of protamine (10 or 15 mg) was given to further reverse heparin. The perioperative target hemoglobin level was approximately 10 g/dL. The decision on mediastinal re-exploration for surgical hemostasis was made by the attending cardiothoracic surgeons based on the comprehensive monitoring of the postoperative patient's hemodynamic condition and aspects of bleeding (bleeding rate, amount, and/or color of drained blood). The general guidelines for re-exploration in our institution included hourly bleeding rates of approximately more than 400 mL/h for 1 hour; more than 300 mL/h for 2 to 3 hours; or more than 200 mL/h for 4 hours despite continued attempts for coagulopathy correction.
2.4. Study endpoints
The primary endpoint was postoperative blood loss measured by cumulative chest tube drainage in the first 24 postoperative hours. Other study endpoints were need for postoperative bleeding-related reoperation and occurrence of postoperative acute kidney injury (AKI). Bleeding-related reoperation was confirmed by manually reviewing hospital records. Postoperative AKI was determined by KDIGO and RIFLE criteria according to creatinine change.[11,12]
2.5. Statistical analysis
Perioperative characteristics such as patient comorbidities or number of coronary arterial anastomoses might bias clinicians in the choice of intraoperative HES administration. To eliminate this bias, patients receiving HES infusion were matched with those not receiving HES infusion based on propensity score. We used 1:N matching rather than 1:1 matching so as to minimize loss of subjects. Logistic regression was used to calculate exposure propensity scores of likelihood of receiving intraoperative HES using all variables listed in Table 1. As variables for outcome analysis, we included previously identified variables such as age greater than 75 years, body mass index (BMI) lower than 25, reoperation, preoperative low hemoglobin, and other clinical factors as risk factors for perioperative bleeding in cardiac surgery.[13,14] After propensity score matching, the balance between the 2 groups was evaluated using standardized difference, variance ratio, and overall distributions. A measure of standardized difference less than 10% was considered to indicate good balance between groups.
Table 1.
Characteristics of matched variables, before and after propensity score matching.
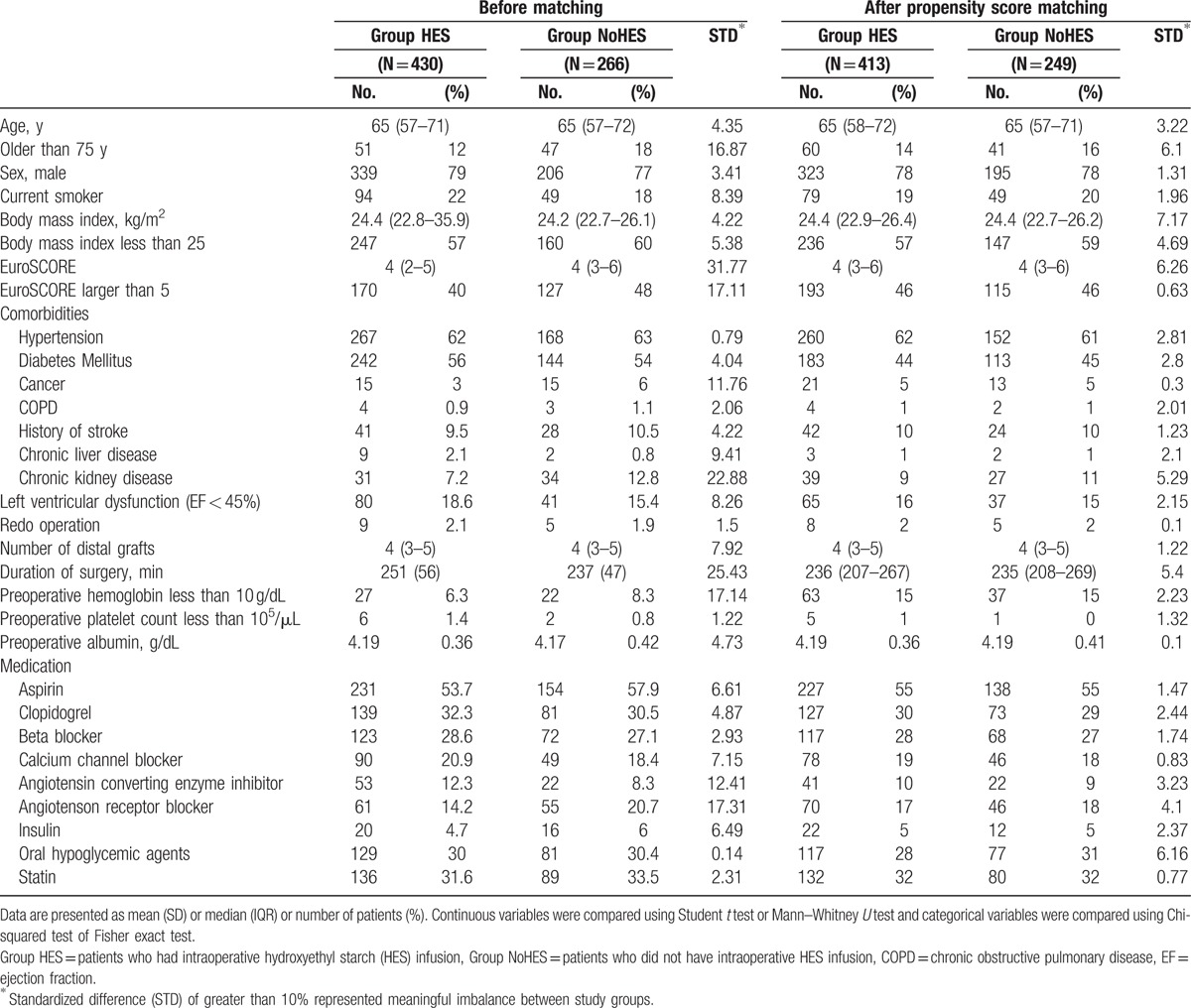
Continuous variables are presented as the mean (standard deviation [SD]) or median (interquartile range), and categorical variables as numbers and percentages. Normality of the continuous variable was assessed by the Kolmogorov–Smirnov test. To compare patient or surgical characteristics between groups, Student t test or the Mann–Whitney U test were used for continuous variables, and the Chi-squared test or Fisher exact test were used for categorical variables. For the comparison of continuous outcomes (e.g., postoperative cumulative blood loss, transfused red blood cells) and to estimate the odds ratio (OR) and 95% confidence interval (CI) for risk of dichotomous postoperative outcome according to HES infusion, we used the generalized estimation equations (GEE) method.
To assess the impacts of independent variables on postoperative AKI, univariate, and multivariable logistic regression models were constructed. Variables with a P value <.1 in univariate analysis were entered into a multivariable logistic regression model. All statistical analyses were performed using the Statistical Analysis System (release 9.3; SAS Institute, Inc., Cary, NC) or IBM SPSS 22 software (SPSS Inc., Chicago, IL). If P < .05, the test was considered to be statistically significant.
3. Results
3.1. Patient and surgical characteristics
Of the 771 patients whose electronic medical records were reviewed, 75 patients who had extracorporeal circulation were excluded (74 had intraoperative cardiopulmonary bypass and 1 had pre- and intraoperative ECMO therapy; Fig. 1). Among the remaining 696 patients, 688 for whom the values of all variables for propensity score matching were available constituted the study cohort. After one-to-many matching according to propensity score, 413 patients who had received intraoperative HES infusion (group HES) were matched with 249 patients who had not (group NoHES), for a total of 662 patients (Fig. 1). Perioperative surgical and clinical characteristics before and after matching are presented in Table 1. The 2 groups had some mismatched variables, including patients older than 75 years, patients with higher EuroSCORE (>5), presence of cancer, chronic kidney disease, or lower preoperative hemoglobin (<10 g/dL), preoperative usage of angiotensin receptor blocker and angiotensin converting enzyme inhibitor, and duration of surgery before propensity score matching (standardized differences >10%). However, there were no significant differences in any variables between groups in the propensity score-matched cohort (Table 1). In the HES group, the median volume of total infused HES was 1000 mL [1000–1500], and the median volume of infused HES according to the patient's body weight was 16 mL/kg [12–21].
Figure 1.
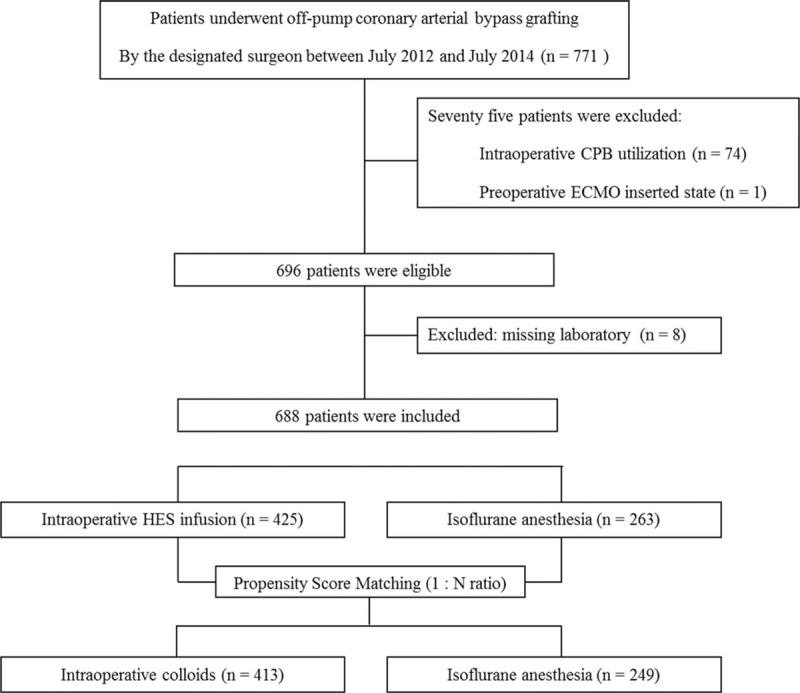
Flow diagram outlining selection of the study population.
3.2. Intraoperative HES and postoperative blood loss/bleeding-related reoperation
Postoperative cumulative blood loss was measured by chest tube drainage during the first 24 hours postoperatively. The median amounts of postoperative blood loss in the 2 groups were comparable (median [IQR]: 525 mL [350–760] in group HES vs. 540 mL [400–670] in group NoHES, P = .203). In-hospital allogenic packed red blood cell transfusions were also comparable between groups (median [IQR]: 3 unit [2–5] in group HES vs. 3 unit [2–4] in group NoHES, P = .243). Of 662 patients, 15 (2.3%) required re-sternotomy for postoperative bleeding. There was no significant difference in the rate of postoperative bleeding-related reoperation between groups (2.9% [12/413) in group HES vs. 1.2% [3/249) in group NoHES, OR 2.44 [95% CI: 0.64–9.34], P = .191).
3.3. Intraoperative HES and postoperative AKI
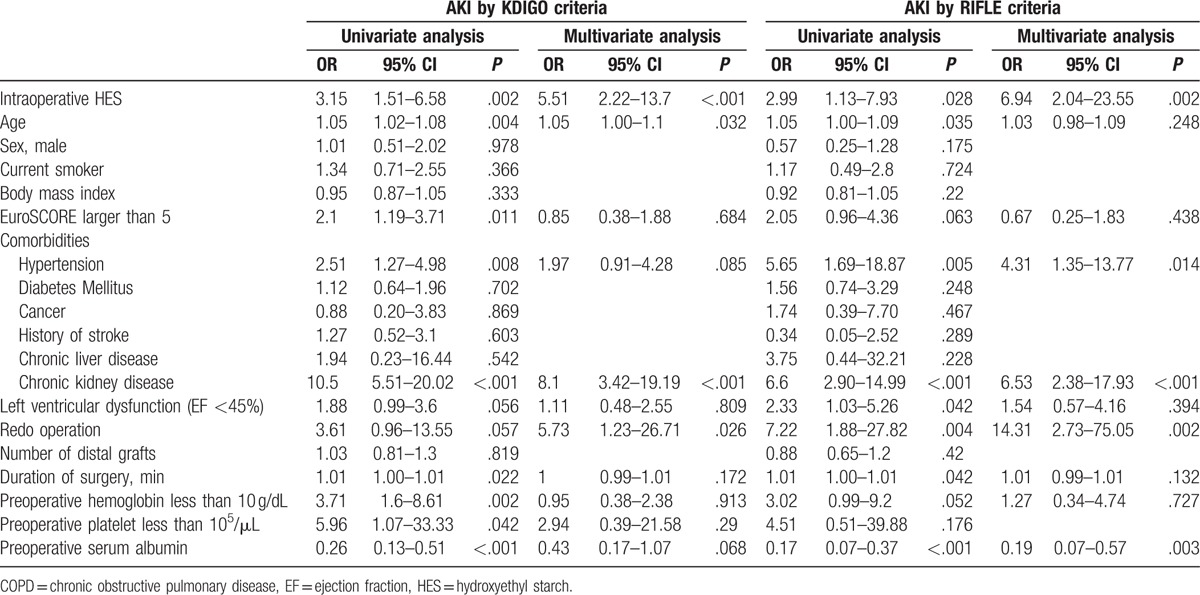
The overall incidence of postoperative AKI was 8% (53/662) by the KDIGO criteria and 4.4% (29/662) by the RIFLE criteria. The incidences of postoperative AKI by both criteria were significantly higher in group HES than group NoHES (AKI by KDIGO: 10.7% [44/413] vs. 3.6% [9/249], OR 3.43 [95% CI: 1.67–7.04], P < .001; AKI by RIFLE: 5.8% [24/413] vs. 2% [5/249], OR 3.32 [95% CI: 1.34–8.24], P = .01, Table 2). In the multivariable logistic regression analysis to identify predictive factors for postoperative AKI, intraoperative use of HES significantly increased the risk of postoperative AKI after adjusting for variables with P < .1 in univariate analysis (OR 5.51 [95% CI: 2.22–13.7], P < .001 for AKI by KDIGO criteria and OR 6.94 [95% CI: 2.04–23.55], P = .002 for AKI by RIFLE criteria, Table 3). Other factors that showed independent association with postoperative AKI were age, presence of preoperative chronic kidney disease, hypertension, redo cardiac operation, and preoperative serum albumin, as shown in Table 3.
Table 2.
Risks of postoperative complications according to the intraoperative hydroxyethyl starch infusion based on matched data.
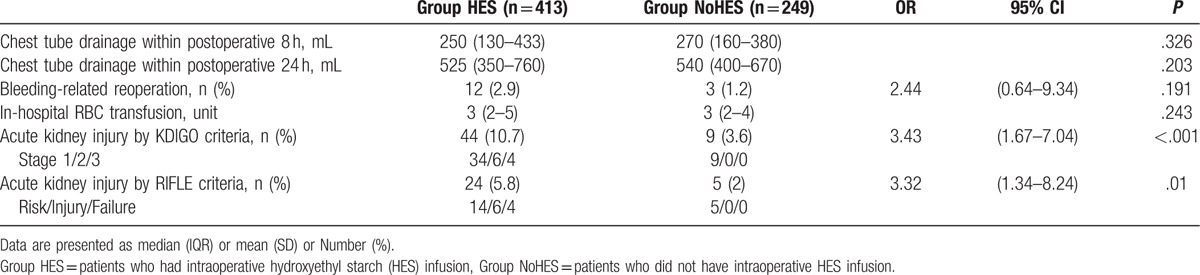
Table 3.
Predictors of postoperative acute kidney injury assessed by the KDIGO and RIFLE criteria.
4. Discussion
In this retrospective propensity-score matched study, we evaluated the effects of the intraoperative use of 6% HES 130/0.4 solution on postoperative blood loss and kidney injury in patients undergoing OPCAB. We found that intraoperative HES did not increase the risk of postoperative cumulative blood loss within the first 24 hours or of bleeding related reoperation. However, use of intraoperative HES was associated with occurrence of postoperative AKI.
Intravenous HES solution was previously widely used as a volume expander to treat hypovolemia in surgical patients. However, since the FDA warning against its use in June 2013, coagulation monitoring has been recommended to address concerns regarding excessive bleeding when using HES during on-pump cardiac surgeries. In a previous meta-analysis of cardiac surgeries involving cardiopulmonary bypass, Navickis et al[15] demonstrated that HES increased blood loss and the need for reoperation due to bleeding. Possible mechanisms of colloid-induced coagulopathy were depletion of circulating coagulation factors, interference of large HES molecules with fibrinogen, coagulation factor VIII and von Willebrand factor, delaying kinetics of fibrin, and impairment of platelet function.[16,17] These conditions resulted in a reduction in clot strength and enhancement of fibrinolysis.[18–20] However, those colloid-induced coagulation derangements were based on analyses including older-generation HES with large molecular weights or highly substituted and high C2:C6 ratio, which undergoes slower metabolism.[15] Moreover, in some studies, the clinical relevance of impairments in thromboelastogram tests for assessing actual bleeding has been questionable.[18,21]
Although earlier meta-analyses of cardiac surgery reported increased risk of postoperative bleeding in patients who received HES,[3,15] a recent meta-analysis including a larger number of recent randomized trials yielded different results.[5] In a recent meta-analysis of cardiac surgery, Jacob et al[5] included 7 OPCAB studies that were excluded in previous meta-analyses and reported that older starches with mean molecular weights greater than 200 kDa increased blood loss and transfusion requirements, but that these adverse effects were not observed with latest-generation tetrastarches (130/0.4). Such differences in the effects of different generation HES formulations on blood loss or coagulation have also been reported in other previous investigations.[2,3,22] In a meta-analysis, Wilkes et al[3] detected lower postoperative blood loss in albumin versus high MMW HES (450 kDa) but not versus medium MMW HES (200 kDa). In a randomized trial by Muralidhar et al,[2] low-molecular weight HES 130/0.4 led to more rapid postoperative recovery of von Willebrand factor (vWF) and less chest tube drainage than medium-molecular weight HES 200/0.5 or gelatin in OPCAB patients. In an in vitro study using thromboelastometry analysis, Roche et al[4] also found that smaller molecular weight-balanced HES preserves coagulation better than large molecular weight starches.
In the present study of OPCAB patients, the intravenous use of 6% HES 130/0.4 was not associated with early postoperative cumulative blood loss or bleeding-related reoperation. Although fewer in number than studies on on-pump cardiac surgery, several investigations have evaluated the impact of HES on perioperative blood loss and coagulation in OPCAB patients. Earlier OPCAB studies using older-generation HES identified increased risk of bleeding or coagulopathy.[23–25] However, a more recent randomized trial in OPCAB patients demonstrated that the use of 6% HES 130/0.4 up to 30 mL/kg did not increase perioperative blood loss compared with the use of crystalloid.[9]
The renal effect of synthetic colloid is another frequently concerned and investigated clinical topic. There is robust evidence of the nephrotoxic effects of colloid solution in critically ill or septic patients.[26–28] The question of whether similar adverse renal effects exist in surgical patients has been investigated in recent reviews and meta-analyses that showed that the use of HES, especially HES 130/0.4, did not increase the risk of postoperative renal dysfunction versus non-starch comparators.[1,6,7,29] However, these meta-analyses mostly included small trials with low event rates, and the authors commented the data insufficiency to identify the renal effects of HES. Moreover, the heterogeneity of the patient samples with various surgical severities included in these trials also raises concerns about the renal safety of HES.
There is only limited data comparing the renal effect of HES compared with crystalloid-only treatment in OPCAB patients. An observational study of 787 OPCAB patients evaluated the effects of perioperative fluid strategy on AKI, but compared different kinds of HES (saline-based versus balanced-solution based), not HES and non-HES.[10] The sample size of another OPCAB study that compared patients treated with HES and crystalloid-only was not calculated to evaluate effects on postoperative AKI, which was a secondary outcome.[9]
Previously, the risk of postoperative acute renal failure was reported to be increased in patients undergoing high-risk surgeries or in patients with coronary arterial disease.[30] In this regard, patients undergoing OPCAB surgery may be a more susceptible population for postoperative kidney injury among surgical populations. Moreover, the elimination half-life of HES was variable according to patient comorbidity and severity of surgery as assessed by means such as inflammation and stress levels of surgery.[31] In our study, variable degrees of kidney injuries (KDIGO stages 1–3) occurred more frequently in the HES group even with the infusion of small volumes of tetra-starch under the recommended dose.
Although the exact mechanism of HES-related kidney injury is poorly understood because of insufficient pathologic evidence, the one suggested mechanism of HES-related kidney injury was tissue uptake of HES molecules by luminal epithelial cells in the proximal tubules, even after treatment with modern HES products with lower molecular weight and degree of substitution. This tissue uptake did not seem to be dose- or time-dependent.[32] Another possible mechanism is a hyperoncotic kidney injury in which glomerular filtration rate decreases secondary to a reduction in filtration fraction.[33] Although most kidney injuries were in the mild stage (KDIGO stage 1), there have been reports on the adverse long-term renal effects of reversible mild renal injury after cardiac surgery.[34] Considering that the perfect prediction of postoperative AKI is difficult and that many patients undergoing OPCAB may require critical care after surgery, the intraoperative use of HES should be considered more cautiously until a clear conclusion is made regarding its renal safety. In addition, careful observation of the occurrence of renal injury would be necessary if it was used inevitably according to the patient's condition in OPCAB patients.
There were several limitations in this retrospective study. First, as this was not a prospective randomized study, there could be hidden bias due to confounding factors. Moreover, a prospective randomized trial is the only reliable way to exclude small relative risks. However, it is difficult to randomize patients for cardiac surgery to receive colloids owing to ethical concerns. We tried to adjust as many of the confounding factors as possible that could affect the outcome occurrence. In particular, we matched most previously reported risk factors of postoperative bleeding[13,14] and AKI[35,36] after cardiac surgery between groups. Moreover, the study was conducted in a homogeneous patient sample who underwent OPCAB by a single surgeon and a single anesthesiologist-in-charge. The results of this retrospective study would be worth investigating before large randomized clinical trials in patients undergoing OPCAB. Although we could not adjust for some factors such as the time point at which the operation was performed (more recent population for NoHES group), we think that the 1 to 2 year difference at which the surgery was performed would not significantly alter the study outcomes because the surgeon and anesthesiologist-in-charge, both of whom were experienced, maintained patient management protocol or surgical techniques during the study periods except for the use of HES solution. Second, because the present study sample consisted of all patients who underwent OPCAB during the study period at our hospital, the effect of HES on postoperative blood loss in patients with preoperative coagulopathy cannot be confirmed. Moreover, the mean volume of infused HES in this study was much lower than the manufacturer-recommended limit of 33 mL/kg, and the effects of HES beyond this recommended limit on postoperative bleeding cannot be clarified. Finally, due to the incomplete follow-up data, we were unable to analyze the long-term effects of intraoperative HES on postoperative survival or renal outcomes. As most of the AKI patients in our samples were in mild stages of renal injury, further studies are needed to confirm the long-term effects of HES in OPCAB patients.
In conclusion, in this retrospective study of patients undergoing OPCAB, we found that third-generation HES suspended in balanced-electrolyte solution did not increase the risk of postoperative bleeding when used within the recommended dose. However, there remains concern regarding an association between intraoperative HES and postoperative AKI. Until confirmative results on renal safety are achieved in further studies, the intraoperative use of 6% HES 130/0.4 should be determined very cautiously during OPCAB.
Footnotes
Abbreviations: AKI = acute kidney injury, BMI = body mass index, CI = confidence interval, ECMO = extracorporeal membrane oxygenation, FDA = Food and Drug Administration, GEE = generalized estimation equations, HES = hydroxyethyl starch, OPCAB = off-pump coronary arterial bypass, OR = odds ratio.
Funding: Support was provided solely by institutional and/or departmental sources.
The authors report no conflicts of interest.
References
- [1].Gillies MA, Habicher M, Jhanji S, et al. Incidence of postoperative death and acute kidney injury associated with i.v. 6% hydroxyethyl starch use: systematic review and meta-analysis. Br J Anaesth 2014;112:25–34. [DOI] [PubMed] [Google Scholar]
- [2].Muralidhar K, Garg R, Mohanty S, et al. Influence of colloid infusion on coagulation during off-pump coronary artery bypass grafting. Indian J Anaesth 2010;54:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wilkes MM, Navickis RJ, Sibbald WJ. Albumin versus hydroxyethyl starch in cardiopulmonary bypass surgery: a meta-analysis of postoperative bleeding. Ann Thorac Surg 2001;72:527–33. discussion 534. [DOI] [PubMed] [Google Scholar]
- [4].Roche AM, James MF, Bennett-Guerrero E, et al. A head-to-head comparison of the in vitro coagulation effects of saline-based and balanced electrolyte crystalloid and colloid intravenous fluids. Anesth Analg 2006;102:1274–9. [DOI] [PubMed] [Google Scholar]
- [5].Jacob M, Fellahi JL, Chappell D, et al. The impact of hydroxyethyl starches in cardiac surgery: a meta-analysis. Crit Care 2014;18:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Van Der Linden P, James M, Mythen M, et al. Safety of modern starches used during surgery. Anesth Analg 2013;116:35–48. [DOI] [PubMed] [Google Scholar]
- [7].Martin C, Jacob M, Vicaut E, et al. Effect of waxy maize-derived hydroxyethyl starch 130/0.4 on renal function in surgical patients. Anesthesiology 2013;118:387–94. [DOI] [PubMed] [Google Scholar]
- [8].Esper SA, Subramaniam K, Tanaka KA. Pathophysiology of cardiopulmonary bypass: Current strategies for the prevention and treatment of anemia, coagulopathy, and organ dysfunction. Semin Cardiothorac Vasc Anesth 2014;18:161–76. [DOI] [PubMed] [Google Scholar]
- [9].Lee JS, Ahn SW, Song JW, et al. Effect of hydroxyethyl starch 130/0.4 on blood loss and coagulation in patients with recent exposure to dual antiplatelet therapy undergoing off-pump coronary artery bypass graft surgery. Circ J 2011;75:2397–402. [DOI] [PubMed] [Google Scholar]
- [10].Kim JY, Joung KW, Kim KM, et al. Relationship between a perioperative intravenous fluid administration strategy and acute kidney injury following off-pump coronary artery bypass surgery: an observational study. Crit Care 2015;19:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Okusa MD, Davenport A. Reading between the (guide)lines—the KDIGO practice guideline on acute kidney injury in the individual patient. Kidney Int 2014;85:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Karkouti K, O’Farrell R, Yau TM, et al. Reducing Bleeding in Cardiac Surgery Research G. Prediction of massive blood transfusion in cardiac surgery. Can J Anaesth 2006;53:781–94. [DOI] [PubMed] [Google Scholar]
- [14].Vuylsteke A, Pagel C, Gerrard C, et al. The Papworth Bleeding Risk Score: a stratification scheme for identifying cardiac surgery patients at risk of excessive early postoperative bleeding. Eur J Cardiothorac Surg 2011;39:924–30. [DOI] [PubMed] [Google Scholar]
- [15].Navickis RJ, Haynes GR, Wilkes MM. Effect of hydroxyethyl starch on bleeding after cardiopulmonary bypass: a meta-analysis of randomized trials. J Thorac Cardiovasc Surg 2012;144:223–30. [DOI] [PubMed] [Google Scholar]
- [16].Nielsen VG. Effects of Hextend hemodilution on plasma coagulation kinetics in the rabbit: role of factor XIII-mediated fibrin polymer crosslinking. J Surg Res 2006;132:17–22. [DOI] [PubMed] [Google Scholar]
- [17].Nielsen VG. Colloids decrease clot propagation and strength: role of factor XIII-fibrin polymer and thrombin-fibrinogen interactions. Acta Anaesthesiol Scand 2005;49:1163–71. [DOI] [PubMed] [Google Scholar]
- [18].Schramko AA, Suojaranta-Ylinen RT, Kuitunen AH, et al. Rapidly degradable hydroxyethyl starch solutions impair blood coagulation after cardiac surgery: a prospective randomized trial. Anesth Analg 2009;108:30–6. [DOI] [PubMed] [Google Scholar]
- [19].Hartog CS, Reuter D, Loesche W, et al. Influence of hydroxyethyl starch (HES) 130/0.4 on hemostasis as measured by viscoelastic device analysis: a systematic review. Intensive Care Med 2011;37:1725–37. [DOI] [PubMed] [Google Scholar]
- [20].de Jonge E, Levi M, Buller HR, et al. Decreased circulating levels of von Willebrand factor after intravenous administration of a rapidly degradable hydroxyethyl starch (HES 200/0.5/6) in healthy human subjects. Intensive Care Med 2001;27:1825–9. [DOI] [PubMed] [Google Scholar]
- [21].Niemi T, Schramko A, Kuitunen A, et al. Haemodynamics and acid-base equilibrium after cardiac surgery: comparison of rapidly degradable hydroxyethyl starch solutions and albumin. Scand J Surg 2008;97:259–65. [DOI] [PubMed] [Google Scholar]
- [22].Gallandat Huet RC, Siemons AW, Baus D, et al. A novel hydroxyethyl starch (Voluven) for effective perioperative plasma volume substitution in cardiac surgery. Can J Anaesth 2000;47:1207–15. [DOI] [PubMed] [Google Scholar]
- [23].Hecht-Dolnik M, Barkan H, Taharka A, et al. Hetastarch increases the risk of bleeding complications in patients after off-pump coronary bypass surgery: a randomized clinical trial. J Thorac Cardiovasc Surg 2009;138:703–11. [DOI] [PubMed] [Google Scholar]
- [24].Yen TS, Chan KC, Cheng YJ. Intraoperative coagulation was more interfered by HES 200/0.5 than normal saline in off-pump coronary artery bypass surgery. J Cardiovasc Surg (Torino) 2008;49:679–84. [PubMed] [Google Scholar]
- [25].Penner M, Fingerhut D, Tacke A. Effect of a new 10% hydroxyethyl starch solution HES/270/0.5 on blood coagulation, blood loss and hemodynamics in comparison with 3.5% PPL. Infusionstherapie 1990;17:314–8. [PubMed] [Google Scholar]
- [26].Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125–39. [DOI] [PubMed] [Google Scholar]
- [27].Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012;367:1901–11. [DOI] [PubMed] [Google Scholar]
- [28].Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012;367:124–34. [DOI] [PubMed] [Google Scholar]
- [29].Raiman M, Mitchell CG, Biccard BM, et al. Comparison of hydroxyethyl starch colloids with crystalloids for surgical patients: A systematic review and meta-analysis. Eur J Anaesthesiol 2016;33:42–8. [DOI] [PubMed] [Google Scholar]
- [30].Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007;107:892–902. [DOI] [PubMed] [Google Scholar]
- [31].Hahn RG, Lyons G. The half-life of infusion fluids: An educational review. Eur J Anaesthesiol 2016;33:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mutter TC, Ruth CA, Dart AB. Hydroxyethyl starch (HES) versus other fluid therapies: effects on kidney function. Cochrane Database Syst Rev 2013;CD007594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moran M, Kapsner C. Acute renal failure associated with elevated plasma oncotic pressure. N Engl J Med 1987;317:150–3. [DOI] [PubMed] [Google Scholar]
- [34].Legouis D, Galichon P, Bataille A, et al. Rapid occurrence of chronic kidney disease in patients experiencing reversible acute kidney injury after cardiac surgery. Anesthesiology 2017;126:39–46. [DOI] [PubMed] [Google Scholar]
- [35].Demirjian S, Schold JD, Navia J, et al. Predictive models for acute kidney injury following cardiac surgery. Am J Kidney Dis 2012;59:382–9. [DOI] [PubMed] [Google Scholar]
- [36].Parolari A, Pesce LL, Pacini D, et al. Risk factors for perioperative acute kidney injury after adult cardiac surgery: role of perioperative management. Ann Thorac Surg 2012;93:584–91. [DOI] [PubMed] [Google Scholar]


