Abstract
The purpose of the current study was to investigate the characteristics of patients with diabetic nephropathy (DN) without diabetic retinopathy (DR).
One hundred four patients with biopsy-proven DN, and 52 patients with diabetes mellitus (DM) without DR who were diagnosed as membranous nephropathy by renal biopsy were retrospectively included. We compared the clinical and laboratory parameters of DN patients with and without DR. Furthermore, among the DM patients without DR, we compared those with DN and with membranous nephropathy.
Among patients with DN, including those with pure DN and DN coexisting with nondiabetic renal disease, compared with patients with DR, those without DR had significantly higher levels of serum albumin and hemoglobin (31.72 ± 7.97 vs 28.49 ± 6.30 g/L, P = .023; 128.11 ± 21.87 vs 113.06 ± 22.03 g/L, P = .001, respectively), and significantly lower level of serum creatinine and prevalence of diabetic neuropathy (148.56 ± 99.19 vs 203.75 ± 145.36 μmol/L, P = .028; 7.30% vs 32.70%, P = .001, respectively). Among patients with pure DN, compared with patients with DR, those without DR had significantly higher level of serum albumin (33.91 ± 5.79 vs 29.32 ± 5.42 g/L, P = .012). Among DM patients without DR, patients with membranous nephropathy had significantly lower levels of serum albumin and serum creatinine, and significantly higher levels of high-density lipoprotein and cholesterol than those with DN.
In conclusion, DN patients without DR may have less serious renal damage and less diabetic complication than those with DR. In the absence of DR, there is still a lack of effective indicators suggesting diabetic nephropathy or nondiabetic glomerulopathy, and renal biopsy is indispensable for diagnosis in such circumstances.
Keywords: diabetes mellitus, diabetic nephropathy, diabetic retinopathy
1. Introduction
Diabetes mellitus (DM) is characterized by chronic hyperglycemia and disturbances in carbohydrate, lipid, and protein metabolism resulting from defects in insulin secretion and/or insulin action. It was estimated that if no urgent action is taken, the number of patients with DM will rise from 415 million in 2015 to 642 million by 2040.[1] About 20% to 40% of patients with DM have diabetic nephropathy (DN).[2] DN is the leading cause of end-stage renal disease in USA, Europe, and Japan.[3] Approximately 4.1 million US adults over 40 years old have diabetic retinopathy (DR).[4] A number of studies showed that DR may be helpful in distinguishing DN in patients with type 2 diabetes mellitus (T2DM) and renal diseases.[5–7] However, the results of these studies are sometimes inconsistent, and DN and DR can appear independently.[8] Characteristics of diabetic nephropathy patients without diabetic retinopathy have not been fully investigated except for a few studies.[8]
DR is a useful indicator for the clinical diagnosis of DN. However, for diabetes patients with proteinuria, if DR is absent, it is sometime difficult to differentiate DN or other glomerulopathy without renal biopsy. To be more specific, membranous nephropathy, the most common type of primary glomerulopathy, has some similar features to DN, including chronic course of the disease, proteinuria with few red blood cells in the urine, and more common in the elderly.
Therefore, in the current study, we compared the clinical and laboratory parameters of DN patients with and without DR; furthermore, among the DM patients without DR, we compared the clinical and laboratory parameters of those with biopsy-proven DN and with membranous nephropathy.
2. Patients and methods
2.1. Patients
One hundred four cases with T2DM in the Department of Nephrology, Peking University First Hospital from September 2005 to December 2015, who were diagnosed as diabetic nephropathy by renal biopsy, were retrospectively recruited in the current study. Renal specimens were evaluated using direct immunofluorescence, light, and electron microscopy by experienced renal pathologists. Classification of DN and pathological scores were evaluated according to the criteria of Tervaert et al.[9] Meanwhile, 52 patients with T2DM without DR, who were diagnosed as membranous nephropathy by renal biopsy in the same period, were recruited. All these patients fulfilled the WHO (1999) type 2 diabetes diagnostic criteria and classification.[10]
Diagnosis of DR was made by trained ophthalmologists according to the International Clinical DR guidelines issued by International Department of Ophthalmology Conference.[11] The results were defined as no apparent retinopathy, nonproliferative diabetic retinopathy, and proliferative diabetic retinopathy.
2.2. Data collection
Clinical data were extracted from the electronic medical records of our hospital, including age, gender, duration of diabetes, duration of hypertension, diabetic neuropathy, diabetic vascular diseases, 24-hours urinary protein excretion, fasting plasma glucose (FPG), HbA1c, hemoglobin, erythrocyte sedimentation rate (ESR), C reactive protein (CRP), albumin, creatinine, triglyceride (TG), total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL). Diabetic complications included diabetic neuropathy and vascular diseases. Peripheral neuropathy was assessed using the validated Toronto Clinical Scoring System (Score >5).[12] For this study, we defined “peripheral vascular disease” as arterial sclerosis of the carotid arteries, upper extremities, abdominal aorta, and other first-order aortic branches. As a method to diagnose peripheral atherosclerosis, ultrasonography of carotid or lower limb arteries was performed to detect the presence of thickening or plaque on the wall of those blood vessels. The research was in compliance with the Declaration of Helsinki and was approved by the ethics committees of Peking University First Hospital. Informed consent was obtained from each patient at renal biopsy.
2.3. Statistical analysis
Variables were represented as means ± SD. Continuous variables were compared by t test or Mann–Whitney U test, while differences of qualitative results were compared using χ2 test, as appropriate. All P values were 2-tailed and considered significant at P < .05. Analyses were performed using SPSS 13.0 (Chicago, IL).
3. Results
3.1. General data
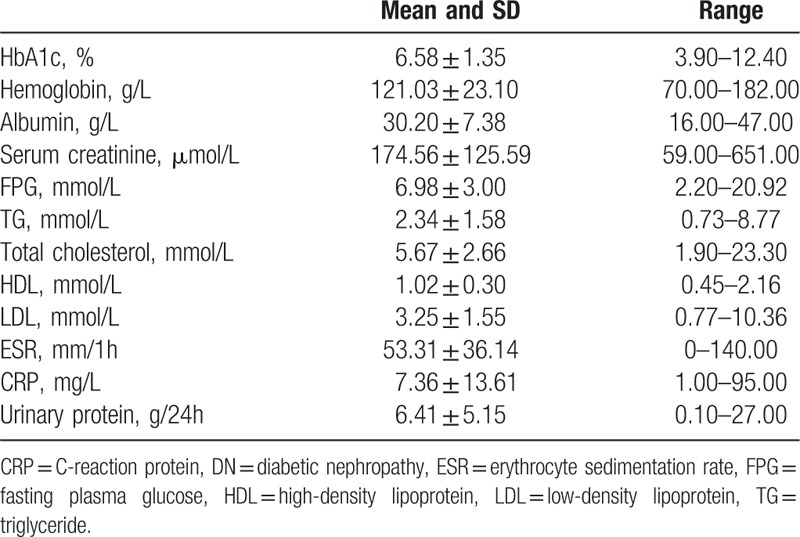
Among the 104 patients with DN, 82 (78.85%) were males and 22 (21.15%) were females, with an age of 50.12 ± 11.65 years old at renal biopsy. The duration of diabetes and hypertension was 8.72 ± 6.58 and 6.22 ± 8.40 years, respectively. Twenty of 104 (19.23%) patients had diabetic neuropathy, 38/104 (36.54%) patients had diabetic vascular diseases. The clinical and laboratory parameters are shown in Table 1.
Table 1.
General data of the patients with DN (n = 104).
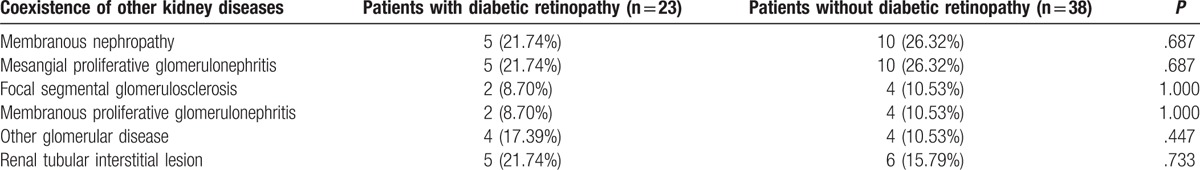
Among these 104 patients with DN, 43 patients had pure DN revealed by renal histopathology, including 26 patients with DR and 17 patients without DR; 61 patients had DN coexisting with nondiabetic kidney disease (NDRD) revealed by renal histopathology, including 23 patients with DR and 38 patients without DR. The coexistence of NDRD is listed in Table 2.
Table 2.
The coexistence of nondiabetic renal disease in patients with diabetic nephropathy.
3.2. Comparisons between DN patients with and without DR
For a DM patient with renal disease who receives renal biopsy, there are 3 possibilities for the renal histopathology, that is, DN, NDRD, and DN coexisting with NDRD.
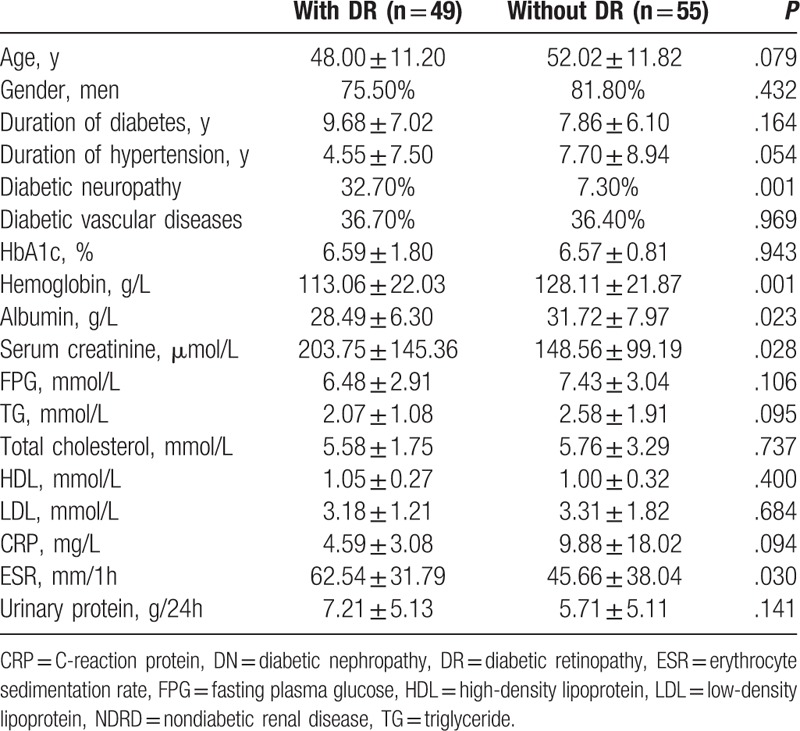
In the total cohort of DN patients, including those with pure DN and DN coexisting with NDRD, we compared characteristics of patients with and without DR. Patients without DR had significantly lower levels of serum creatinine at renal biopsy (148.56 ± 99.19 vs 203.75 ± 145.36 μmol/L, P = .028) than those with DR. The prevalence of diabetic neuropathy in patients without DR was significantly lower than that in patients with DR (7.30% vs 32.70%, P = .001). Compared with patients with DR, those without DR had significantly higher levels of albumin and hemoglobin (31.72 ± 7.97 vs 28.49 ± 6.30 g/L, P = .023; 128.11 ± 21.87 vs 113.06 ± 22.03 g/L, P = .001, respectively) (Table 3).
Table 3.
Comparisons between DN patients (including those with pure DN and DN coexisting with NDRD) with and without DR.
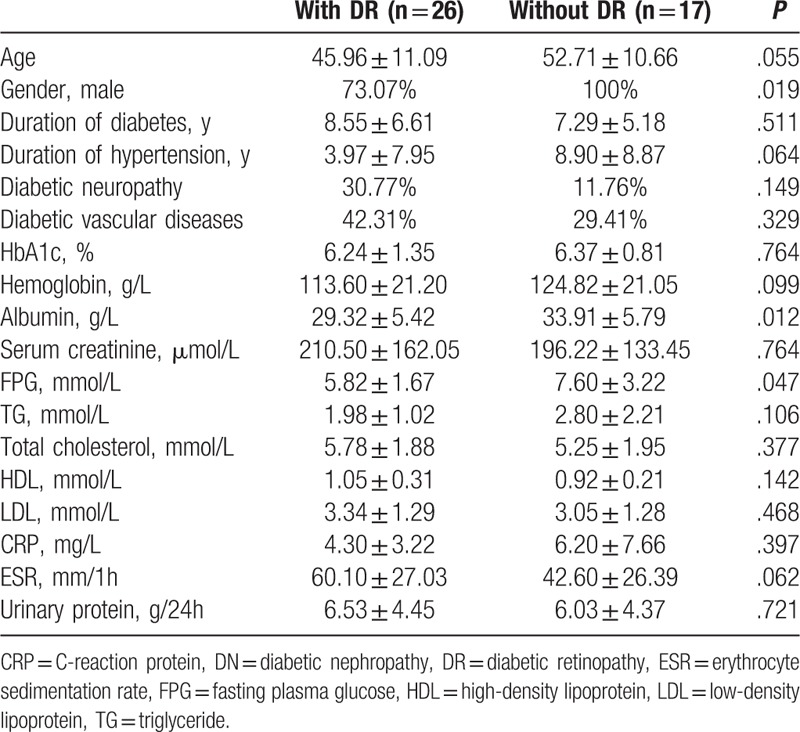
Then, we compared pure DN patients, that is, without the coexistence of NDRD, with DR and without DR. Compared with DN patients with DR, those without DR had significantly higher prevalence of male (100% vs 73.07%, P = .019), and significantly higher levels of albumin and FPG (33.91 ± 5.79 vs 29.32 ± 5.42 g/L, P = .012; 7.60 ± 3.22 vs 5.82 ± 1.67 mmol/L, P = .047, respectively) (Table 4). We also compared the renal pathological characteristics between DN patients with and without DR, and found no significant difference between these 2 subgroups of patients, including classification of renal pathology, tubular atrophy, interstitial fibrosis, podocyte injury and deposition of immunoglubulins, and complements in immunofluorescence.
Table 4.
Comparisons between pure DN patients with DR and without DR.
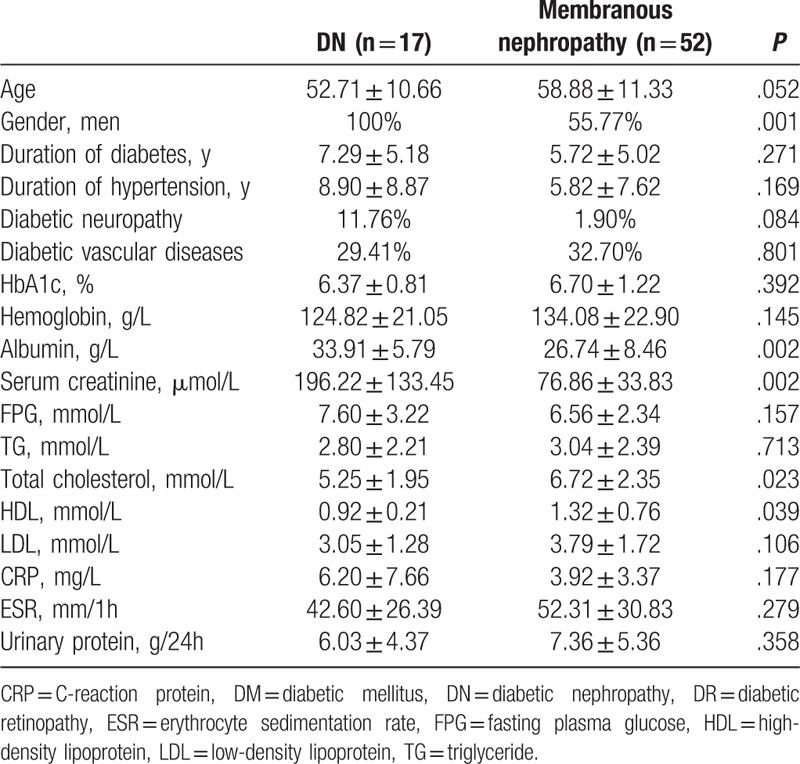
3.3. Comparisons between patients with pure DN and patients with membranous nephropathy among DM patients without DR
As mentioned above, for diabetes patients with proteinuria and without DR, if renal histology is not available, it is sometime difficult to differentiate DN or other glomerulopathy, especially membranous nephropathy. Therefore, we further compared these 2 groups of patients, expecting to find some clues for differential diagnosis. It was found that the prevalence of male in patients with DN was significantly higher than that in patients with membranous nephropathy (100% vs 55.77%, P = .001). Compared with patients with membranous nephropathy, those with DN had significantly higher levels of albumin and serum creatinine at renal biopsy (33.91 ± 5.79 vs 26.74 ± 8.46 g/L, P = .002; 196.22 ± 133.45 vs 76.86 ± 33.83 μmol/L, P = .002, respectively), and significantly lower levels of total cholesterol and HDL (5.25 ± 1.95 vs 6.72 ± 2.35 mmol/L, P = .023; 0.92 ± 0.21 vs 1.32 ± 0.76 mmol/L, P = .039, respectively) (Table 5).
Table 5.
Comparisons between patients with pure DN and with membranous nephropathy among DM patients without DR.
4. Discussion
DN and DR are the 2 most important diabetic microvascular complications, and they have similar pathological basis. Previous studies found that DR is an important predictor for DN.[13] However, there were still many conditions that DN was not associated with DR, and the incidence of fundus lesions was inconsistent in different studies.[14–18] Characteristics of DN patients without DR are not fully clear yet.
In this study, we analyzed the clinical and laboratory feature of DN patients without DR, in comparison with the “classical” DN, that is, those with DR. Compared with patients with DR, patients without DR had a lower level of serum creatinine and lower prevalence of diabetic neuropathy, suggesting that patients without DR had less serious renal damage and less diabetic complications. It was consistent with the study by Katulanda et al,[19] who found that in DM patients, peripheral neuropathy was associated with DR. We also found that the level of albumin in patients without DR was significantly higher than that in patients with DR. Although the difference in proteinuria was not significant, DR was associated with the duration of diabetes [19]; chronic metabolic disorder of diabetes would accelerate the decomposition of albumin resulting in lower level of albumin.
Another clinically relevant issue is that for diabetes patients with proteinuria, if DR is absent, it is sometime difficult to differentiate DN from other glomerulopathy, especially membranous nephropathy, without renal biopsy. In particular, DN and membranous nephropathy have some similar clinical characteristics, as mentioned above. Therefore, we compared the clinical and laboratory parameters of those with DN and membranous nephropathy in DM patients without DR, expecting to find some clues for differential diagnosis of these 2 circumstances in the case that renal biopsy is unavailable. We found that among DM patients without DR, compared with DN patients, patients with membranous nephropathy had significantly higher levels of HDL and cholesterol, which was consistent with the characteristics of membranous nephropathy. Meanwhile, the level of creatinine in patients with DN was significantly higher than that in patients with membranous nephropathy. However, these differences were relatively minor despite the statistical difference, and not robust enough to provided effective indicators for the differential diagnosis of these 2 entities.
The main limitation of our study was the selection bias. In the routine clinical practice in our center, we often made the diagnosis of diabetic nephropathy clinically when DM patients had diabetic retinopathy and proteinuria, and therefore this subgroup of patients seldom received renal biopsy. Patients with DM usually received renal biopsy when they were suspected to have NDRD. Therefore, our results should be confirmed by a larger prospective study.
In conclusion, DN patients without DR may have less serious renal damage and less diabetic complication than those with DR. In the absence of DR, there is still a lack of effective indicators suggesting DN or nondiabetic glomerulopathy, and renal biopsy is indispensable for diagnosis in such circumstances.
Footnotes
Abbreviations: CRP = C-reaction protein, DM = diabetes mellitus, DN = diabetic nephropathy, DR = diabetic retinopathy, ESR = erythrocyte sedimentation rate, FPG = fasting plasma glucose, HDL = high-density lipoprotein, LDL = low-density lipoprotein, NDRD = nondiabetic renal disease, TG = triglyceride.
X-QL and XZ were responsible for the recruitment of patients, data collection and analyzing the results, interpreted the results and wrote the paper. MC and M-HZ conceived and directed the study. All authors reviewed the manuscript.
This study is supported by two grants from the National Natural Science Fund (No. 81425008 and No. 81621092), a grant by the University of Michigan Health System and Peking University Health Sciences Center Joint Institute for Translational and Clinical Research, and the grant from National Key Research and Development Program (No. 2016YFC1305405).
The authors have no conflicts of interest to disclose.
References
- [1].The International Diabetes Federation (2015) International Diabetes Federation's 7th edition of the Diabetes atlas. Available at: http://www.diabetesatlas.org/key-messages.html. Accessed December 1, 2015. [Google Scholar]
- [2].KDOQI. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007;49(suppl 2):S12–54. [DOI] [PubMed] [Google Scholar]
- [3].Kikkawa R, Koya D, Haneda M. Progression of diabetic nephropathy. Am J Kidney Dis 2003;41(suppl 1):S19–21. [DOI] [PubMed] [Google Scholar]
- [4].Kempen JH, O’Colmain BJ, Leske MC. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol 2004;122:552–63. [DOI] [PubMed] [Google Scholar]
- [5].Parving HH, Gall MA, Skøtt P. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int 1992;41:758–62. [DOI] [PubMed] [Google Scholar]
- [6].Al-Rubeaan K, Youssef AM, Subhani SN. Diabetic nephropathy and its risk factors in a society with a type 2 diabetes epidemic: a Saudi National Diabetes Registry-based study. PLoS One 2014;9:e88956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].He F, Xia X, Wu XF. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia 2013;56:457–66. [DOI] [PubMed] [Google Scholar]
- [8].Wolf G, Müller N, Mandecka A, et al. Association of diabetic retinopathy and renal function in patients with types 1 and 2 diabetes mellitus. Clin Nephrol 2007;68:81–6. [DOI] [PubMed] [Google Scholar]
- [9].Tervaert TW, Mooyaart AL, Amann K. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010;21:556–63. [DOI] [PubMed] [Google Scholar]
- [10].Report of a WHO Consultation, Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1 Diagnosis and classification of diabetes mellitus. World Health Organization, Department of Noncommunicable Disease Surveillance, Geneva, 1999. [Google Scholar]
- [11].Wilkinson CP, Ferris FL 3rd, Klein RE. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–82. [DOI] [PubMed] [Google Scholar]
- [12].Perkins BA, Olaleye D, Zinman B, et al. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care 2001;24:250–6. [DOI] [PubMed] [Google Scholar]
- [13].Rossing P, Hougaard P, Parving H-H. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care 2002;25:859–64. [DOI] [PubMed] [Google Scholar]
- [14].Kotlarsky P, Bolotin A, Dorfman K. Link between retinopathy and nephropathy caused by complications of diabetes mellitus type 2. Int Ophthalmol 2015;35:59–66. [DOI] [PubMed] [Google Scholar]
- [15].Zhou J, Chen X, Xie Y, et al. A differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases. Nephrol Dial Transplan 2008;23:1940–5. [DOI] [PubMed] [Google Scholar]
- [16].Serra A, Romero R, Bayés B, et al. Is there a need for changes in renal biopsy criteria in proteinuria in type 2 diabetes? Diabetes Res Clin Pract 2002;58:149–53. [DOI] [PubMed] [Google Scholar]
- [17].Soni SS, Gowrishankar S, Kishan AG, et al. Non diabetic renal disease in type 2 diabetes mellitus. Nephrology (Carlton) 2006;11:533–7. [DOI] [PubMed] [Google Scholar]
- [18].Pham TT, Sim JJ, Kujubu DA, et al. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol 2007;27:322–8. [DOI] [PubMed] [Google Scholar]
- [19].Katulanda P, Ranasinghe P, Jayawardena R. Prevalence of retinopathy among adults with self-reported diabetes mellitus: the Sri Lanka diabetes and Cardiovascular Study. BMC Ophthalmol 2014;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]


