Introduction
KEY TEACHING POINTS
|
Brugada syndrome is known to be a life-threatening disease provoking lethal arrhythmias such as ventricular tachycardia/ventricular fibrillation (VF), but non–implantable cardioverter-defibrillator (ICD) treatment strategy remains a topic of discussion.1 Substrate-based catheter ablation of Brugada syndrome has been reported to be effective,2, 3 but much is still unknown, for example, the relationship between local abnormal endocardial potentials and ablation results.
Case report
A 37-year-old man was referred to our hospital after resuscitation from VF due to Brugada syndrome and an ICD was implanted for secondary prevention. A second VF attack occurred 8 months later, and oral bepridil (100 mg/d; a drug shown to have some success in reducing electrical storms in patients with Brugada syndrome4, 5, 6) was initiated. After a year and half of remission, there was a recurrence of VF, and over the next 6 months, there were 9 appropriate ICD shocks (all for VF). Both type 1 and type 2 Brugada electrocardiographic (ECG) patterns were observed with fluctuating amplitude at the time of ICD implantation, but the coved-type ECG became constant as the VF attacks increased. An increased dose of bepridil (200 mg/d) and cilostazol (200 mg/d) failed to control VF and VF progressed to electrical storms (maximum 3 times in a day). At that point, the decision was made to perform catheter ablation for the arrhythmia.
Premature ventricular contraction (PVC) triggering VF had never been observed, and substrate ablation in the endocardial right ventricular outflow tract (RVOT) was attempted with preparations for backup epicardial ablation, and the subxiphoid area was draped in a sterile fashion. CARTO 3 (Biosense Webster, Diamond Bar, CA) mapping in the RVOT showed low-voltage areas (<1.5 mV with bipolar mapping) beneath the pulmonary artery valve circumferentially, and also in the lower anterior aspect, with irregularly shaped extensions to the lateral and septal areas. Fractionated or delayed potentials were observed mostly within these low-voltage areas including the posterior area, all of which were targeted for ablation (Figure 1). A ThermoCool SF (Biosense Webster, Diamond Bar, CA) catheter was used, and radiofrequency (RF) energy within the range of 30–35 W was delivered. During the procedure, VF occurred 5 times. Of these, 4 were provoked during catheter mapping and the fifth occurred during RF application at an RVOT anterior site. Circumferential and zonal ablation was performed in the RVOT with a total application count of 59 times (55 minutes) and a total energy of 96,186 J. The 12-lead ECG changed gradually as the ablation procedure progressed. After the final RF application, the coved-type morphology in lead V1 had disappeared and ST-segment elevation in lead V2 had diminished (Figure 2). The last VF occurred in the middle of the procedure, after which no further VF was observed. We ended the procedure when all the fractionated or delayed potentials were ablated in a manner similar to when substrate ablation is conducted in the epicardium,2 but an additional drug stress test was not performed because of the prolonged procedure time and the high frequency of necessary defibrillation shocks during the procedure.
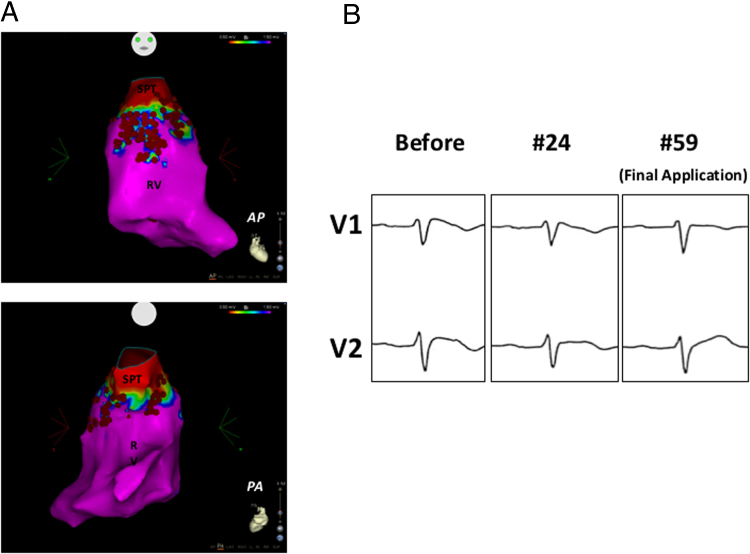
Figure 1.
A: CARTO mapping in the endocardial right ventricular outflow tract showed low-voltage areas beneath the pulmonary artery valve, with discrete irregularly shaped extensions mostly in the anterior surface, but with some to the lateral and septal sides. B: Electrograms from areas marked a to c in panel A. Fractionated and delayed potentials were observed mostly within the low-voltage area, all of which were targeted as ablation sites. ABL = ablation catheter; AP = anterior-posterior view; d = distal; p = proximal; PA = posterior-anterior view; RV = right ventricle; SPT = sinus of pulmonary trunk.
Figure 2.
All the delayed and fractionated potentials around the right ventricular outflow tract were targeted and ablated. Red tags show ablation points. Application power was limited to 30 W except for some septal sites where it was 35 W, and an SL0 sheath was used to maintain firm contact (A). Typical coved-type electrocardiogram displayed at the beginning of the procedure (Before). ST-segment elevation gradually diminished as ablation proceeded Radio frequency catheter ablation (RFCA #24). We ended the procedure with the disappearance of the coved-type electrocardiographic pattern, the improvement of ST-segment elevation in lead V2, and the cessation of ventricular fibrillation occurrences (Final Application (#59)) (B). AP = anterior-posterior view; PA = posterior-anterior view.
The patient was discharged 2 days after the ablation procedure without complication. The minimum ST level was observed 6 weeks after the procedure (Figure 3). Cilostazol (200 mg/d) was used just after the ablation procedure, but was tapered and discontinued 2 months after the procedure. During the 6-month observation period after the procedure, an appropriate ICD shock for VF was experienced once within 5 months, and a subsequent disopyramide stress test for the evaluation of the remaining arrhythmogenicity proved positive with the appearance of the coved-type ECG patterns in lead V1. However, no further VF storms have been observed since the ablation procedure up to the present time.
Figure 3.
ST level showed changes with daily variance over the 2 days after the ablation procedure until discharge. Antiarrhythmic drugs were discontinued 14 days after the procedure. Minimum ST level was observed 42 days after the procedure. The coved-type electrocardiographic pattern was not observed during the half year of follow-up.
Discussion
For 1 particular case, we showed the effectiveness of endocardial-only ablation in preventing Brugada syndrome–induced VF storms with debilitating ICD shocks in the antiarrhythmic drug–free state. However, recurrence of VF once and the appearance of the Brugada ECG pattern on pharmacological challenge during follow-up show the limitations of our method in curing the underlying substrate of the arrhythmia.
Endocardial substrate ablation
In our case, PVCs triggering VF had never been detected before the ablation procedure,7, 8 so a substrate-modifying ablation strategy was selected. Nademanee et al2 performed an initial substrate-based catheter ablation of Brugada syndrome in the epicardium. Sunsaneewitayakul et al3 reported endocardium substrate ablation by identifying the late activation zones (LAZs) in the RVOT using noncontact mapping (EnSite 3000, St. Jude Medical, Minneapolis, MN), and the LAZs were targeted as ablation sites. In our case, endocardial ablation was performed targeting the fractionated and/or delayed potentials recorded by conventional 3-dimensional contact mapping. In the literature, it has been reported that these local abnormal potentials could be detected in the endocardial site in the RVOT,9 but ablation targeting these potentials is rarely adopted, probably because these potentials are not always detected in patients with Brugada syndrome, and its efficacy is still uncertain. Although Shah et al10 reported a case of endocardial ablation with Brugada syndrome, target locations in the RVOT were determined on the basis of pace mapping using the clinical PVC as reference, and local abnormal potentials were not mentioned. Our case demonstrated the potential effectiveness of local electrogram-guided endocardial-only ablation with conventional 3-dimensional contact mapping in Brugada syndrome for preventing VF storms.
Endocardial fractionated potentials were detected not only in the anterior aspect of the RVOT but also in the lateral, septal, and posterior aspects in our case. These expanded abnormal potentials in the RVOT may have a relationship to the LAZ, because LAZs were also present in various locations of the RVOT, and the existence of fractionated potentials inside the LAZ has been reported in patients with frequent VF by Sunsaneewitayakul et al.3
Epicardial arrhythmogenic substrate
Epicardial mapping was not performed in this case, because sufficient effect was achieved by the endocardial approach alone in this subemergent situation. As a result, the relationship between epicardial arrhythmogenicity and the endocardial local abnormal potentials was not examined in this procedure. In our case, most of the delayed potentials were observed in or near the low-voltage area. This is similar to the distribution of delayed potentials in epicardial sites as previously reported.2 There remains a possibility that endocardial fractionated and/or delayed potential may reflect the far-field potential of the epicardial abnormal potentials and that the ablation lesion from the endocardium might have affected the arrhythmogenic sites in the epicardium. In this regard, endocardial ablation in Brugada syndrome may require the formation of transmural lesions, such as those performed with the Thermocool SF catheter with support of the SL0 sheath (St. Jude Medical Minneapolis, MN). Thickness of the RVOT wall is estimated to be approximately 3–6 mm,11 and this anatomical variation may have an impact on effectiveness in endocardial-only ablation.11
End point of the ablation procedure
We successfully met our end point of ablating all fractionated and delayed potentials, noted the near normalization of the Brugada ECG pattern and the disappearance of VF during the procedure. Within a half year of the procedure, VF recurred once in an antiarrhythmic drug–free state, and a subsequent disopyramide stress test produced the coved-type Brugada ECG pattern. These findings indicate that arrhythmogenicity of Brugada syndrome in this patient was not fully eradicated. This might be a limitation of endocardial-only ablation in that the remaining arrhythmogenic substrate in the epicardium could not be detected from the endocardium. That said, endocardial ablation has an advantage over epicardial-side ablation, which can cause severe complications.12 The unipolar voltage map (<5.5 mV) showed a more extended low-voltage area in the RVOT than did the bipolar voltage map, and this might be related to the remaining arrhythmogenic substrate.13
We will consider performing epicardial ablation on this patient, should he have a recurrent increase in VF episodes as we follow him up.
References
- 1.Antzelevitch C., Brugada P., Borggrefe M. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 2.Nademanee K., Veerakul G., Chandanamattha P., Chaothawee L., Ariyachaipanich A., Jirasirirojanakorn K., Likittanasombat K., Bhuripanyo K., Ngarmukos T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium: clinical perspective. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 3.Sunsaneewitayakul B., Yao Y., Thamaree S., Zhang S. Endocardial mapping and catheter ablation for ventricular fibrillation prevention in Brugada syndrome. J Cardiovasc Electrophysiol. 2012;23:s10–s16. doi: 10.1111/j.1540-8167.2012.02433.x. [DOI] [PubMed] [Google Scholar]
- 4.Shinohara T., Ebata Y., Ayabe R., Fukui A., Okada N., Yufu K., Nakagawa M., Takahashi N. Combination therapy of cilostazol and bepridil suppresses recurrent ventricular fibrillation related to J-wave syndromes. Heart Rhythm. 2014;11:1441–1445. doi: 10.1016/j.hrthm.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Murakami M., Nakamura K., Kusano K.F. Efficacy of low-dose bepridil for prevention of ventricular fibrillation in patients with Brugada syndrome with and without SCN5A mutation. J Cardiovasc Pharmacol. 2010;56:389–395. doi: 10.1097/FJC.0b013e3181f03c2f. [DOI] [PubMed] [Google Scholar]
- 6.Aizawa Y., Yamakawa H., Takatsuki S. Efficacy and safety of bepridil for prevention of ICD shocks in patients with Brugada syndrome and idiopathic ventricular fibrillation. Int J Cardiol. 2013;168:5083–5085. doi: 10.1016/j.ijcard.2013.07.187. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa E., Takagi M., Tatsumi H., Yoshiyama M. Successful radiofrequency catheter ablation for electrical storm of ventricular fibrillation in a patient with Brugada syndrome. Circ J. 2008;72:1025–1029. doi: 10.1253/circj.72.1025. [DOI] [PubMed] [Google Scholar]
- 8.Haïssaguerre M., Extramiana F., Hocini M. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation. 2003;108:925–928. doi: 10.1161/01.CIR.0000088781.99943.95. [DOI] [PubMed] [Google Scholar]
- 9.Postema P.G., van Dessel PFHM, de Bakker J.M.T., Dekker L.R.C., Linnenbank A.C., Hoogendijk M.G., Coronel R., Tijssen J.G.P., Wilde A.A.M., Tan H.L. Slow and discontinuous conduction conspire in Brugada syndrome: a right ventricular mapping and stimulation study. Circ Arrhythm Electrophysiol. 2008;1:379–386. doi: 10.1161/CIRCEP.108.790543. [DOI] [PubMed] [Google Scholar]
- 10.Shah A.J., Hocini M., Lamaison D., Sacher F., Derval N., Haissaguerre M. Regional substrate ablation abolishes Brugada syndrome. J Cardiovasc Electrophysiol. 2011;22:1290–1291. doi: 10.1111/j.1540-8167.2011.02054.x. [DOI] [PubMed] [Google Scholar]
- 11.De Ponti R., Ho S.Y. Mapping of right ventricular outflow tract tachycardia/ectopies: activation mapping versus pace mapping. Heart Rhythm. 2008;5:345–347. doi: 10.1016/j.hrthm.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Killu A.M., Friedman P.A., Mulpuru S.K., Munger T.M., Packer D.L., Asirvatham S.J. Atypical complications encountered with epicardial electrophysiological procedures. Heart Rhythm. 2013;10:1613–1621. doi: 10.1016/j.hrthm.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Polin G.M., Haqqani H., Tzou W., Hutchinson M.D., Garcia F.C., Callans D.J., Zado E.S., Marchlinski F.E. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8:76–83. doi: 10.1016/j.hrthm.2010.09.088. [DOI] [PubMed] [Google Scholar]