Figure 1.
Homozygous Mutations in PLAA Causes a Severe, Infantile Neurodysfunction Disorder
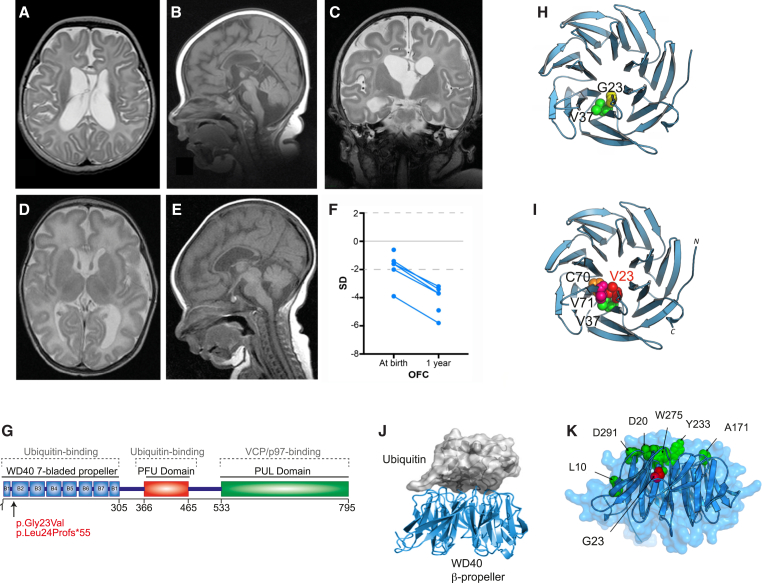
(A–E) MRI images of individuals A-IV-8 (A–C, aged 3 months) and A-IV-1 (D, E, aged 3 weeks). Axial (A, D), sagittal (B, E), coronal (C), T1-weighted (B, D, E), T2-weighted (A, C). Widespread T2-hyperintensity throughout the white matter and simplified gyral pattern frontally are evident. Ventricles and occipital horns are asymmetrically dilatated (A, C). Thinning of corpus callosum is evident (B, E).
(F) Z-scores of the occipital frontal circumference (OFC) of affected individuals at birth and around a year highlight progressive microcephaly.
(G) The mutations fall in the first exon, within the WD40 repeat domain of PLAA.
(H) Homology model of the WD40 β-propeller domain of human PLAA, based upon the crystal structure of yeast Doa1; Gly23 is buried within the inner-most β strand 1 within blade 2 where it supports hydrophobic interaction with Val37.
(I) Mutant Val23 is predicted to destabilize structure likely due to steric clashes between its side chain Cϒ1 atom with spatially proximal residues in blade 3 (labeled).
(J) Crystal structure of the yeast PLAA homolog Doa1 N-terminal WD40 β-propeller (cyan) in complex with ubiquitin (white surface), adapted from Pashkova et al.22
(K) Location of experimentally defined key residues for ubiquitin-binding (green) with respect to p.Gly23Val (red) shown mapped on the 3D model of human PLAA WD40 β-propeller.
See also Figure S1.