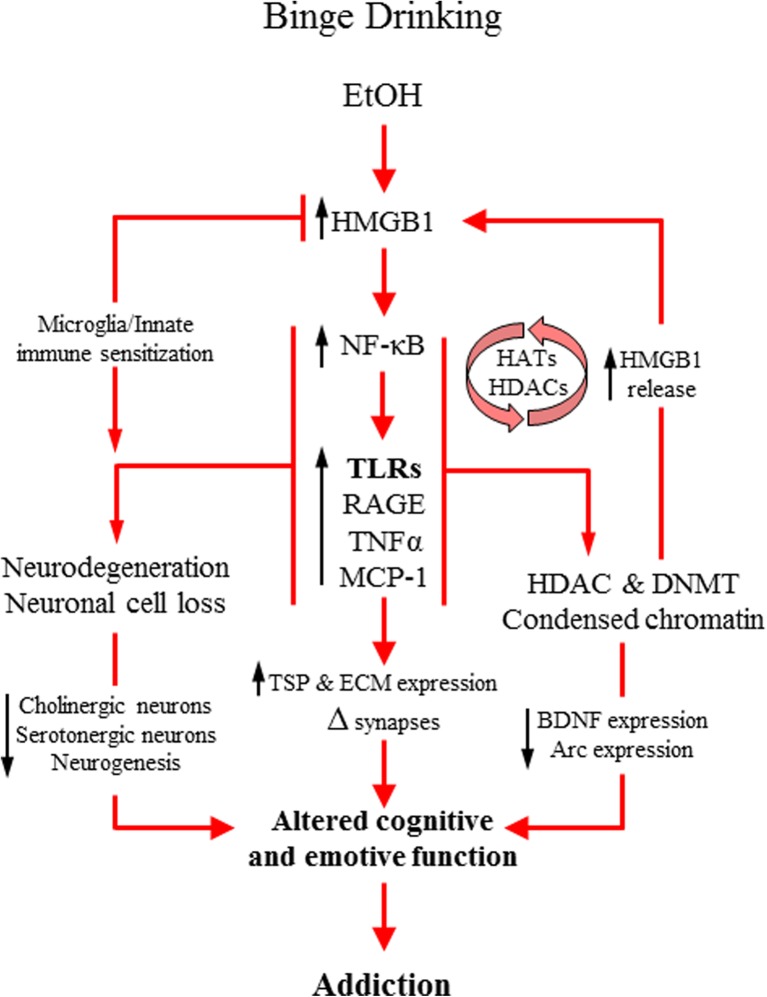
Fig. 4.
Simplified schematic depicting mechanisms of alcohol-induced alterations to brain plasticity. Alcohol binge drinking and abuse increases expression of the endogenous innate immune receptor agonist high-mobility group box 1 (HMGB1) leading to activation of the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and subsequent induction of proinflammatory cytokines and oxidases (Crews et al. 2013; Qin and Crews 2012a; Vetreno and Crews 2012, 2015; Vetreno et al. 2016). This also leads to increased expression of toll-like receptors (TLRs) and the receptor for advanced glycation end-products (RAGE) (Crews et al. 2013; Vetreno and Crews 2012, 2015; Vetreno et al. 2013), which are receptors for HMGB1. Activation of this signaling pathways leads to the establishment of positive loops of amplification that persist during abstinence from alcohol (Crews and Vetreno 2016) and alter synapse formation through increased expression of thrombospondins (Risher et al. 2015) and alterations in extracellular matrix proteins (Coleman et al. 2014). Concomitantly, innate immune induction causes epigenetic modifications that induce further release of HMGB1 (Zou and Crews 2014) that contribute to positive loops of amplification as well as alterations in synaptic plasticity molecules (Pandey et al. 2015; Sakharkar et al. 2016) that contribute to alterations in cognitive and emotive functioning. Innate immune driven epigenetic modifications might also lead to microglial alterations resulting in the reprogramming of the innate immune system. Innate immune induction also causes neurodegeneration (Braun and Crews 2000; Crews et al. 2000) and the loss of neuron-specific cell populations (Boutros et al. 2015; Vetreno et al. 2014; Vetreno and Crews 2015; Vetreno et al. 2016) that might also contribute to the dysfunctional cognitive and affective states observed in addiction (Crews and Vetreno 2016)