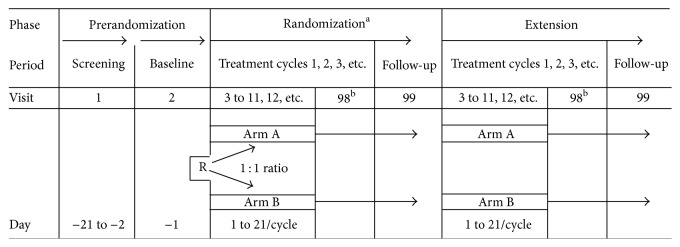
Figure 1.
R: randomization. Arm A: eribulin mesylate 1.4 mg/m2 IV on Days 1 and 8, every 21 days. Arm B: dacarbazine IV on Day 1, every 21 days. The starting dose must be selected from one of the following doses: 850 mg/m2, 1,000 mg/m2, or 1,200 mg/m2. aThe randomization phase will end at the time of data cut-off for the primary analysis when the target number of events has been observed. All subjects still on treatment with study treatment or in survival follow-up will then enter the extension phase. bOff-treatment visit.