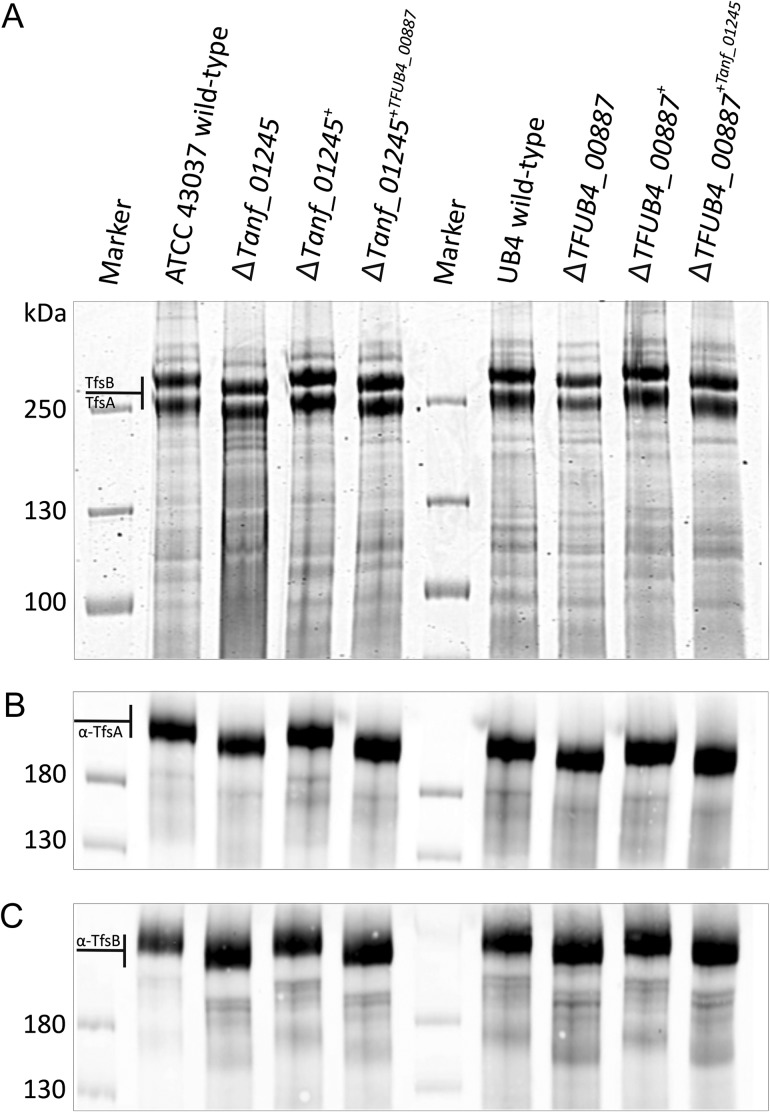
Fig. 2.
SDS-PAGE and Western immunoblot analyses of T. forsythia ATCC 43037 and T. forsythia UB4 wild-type and mutants. (A) CBB staining of crude cell extracts from T. forsythia ATCC 43037 wild-type, ΔTanf_01245 mutant, reconstituted mutant ΔTanf_01245+ and cross-complemented mutant ΔTanf_01245+TFUB4_00887 after separation on a 7.5% SDS-PA gel. The S-layer glycoproteins (labeled TfsA and TfsB) are indicated and the down-shift resulting from the loss of the Pse5Am7Gra residue can be observed in the deletion mutant and in the cross-complemented mutant, while in the reconstituted strain the bands are up-shifted again to wild-type level. The same migration profiles could be observed for T. forsythia UB4 wild-type, ΔTFUB4_00887 mutant, reconstituted mutant ΔTFUB4_00887+ and cross-complemented mutant ΔTFUB4_00887+Tanf_01245. PageRuler Plus Prestained Protein Ladder (Thermo Fisher Scientific) was used as a protein molecular weight marker. The S-layer glycoprotein bands were further processed for MS analyses. Western immunoblots probed with anti-TfsA antiserum (B) and anti-TfsB antiserum (C) confirmed the identity of the S-layer glycoproteins in all analyzed T. forsythia species. PageRuler Prestained Protein Ladder (Thermo Fisher Scientific) was used as a molecular weight marker.