Abstract
Introduction
Single-ventricle patients with elevated pulmonary vascular resistance (PVR) or end-diastolic pressure (EDP) are excluded from undergoing total cavopulmonary connection (TCPC). However, a subset of patients deemed acceptable risk experience prolonged length of stay (LOS) after TCPC. Routine assessment of ventricular function has been inadequate in identifying these high risk patients. Speckle-tracking echocardiography (STE) is a novel method for assessment of myocardial deformation that may be useful in single-ventricle patients. The aim of this study was to perform a contemporary pre-operative risk assessment for prolonged LOS to determine if STE improves risk stratification prior to TCPC.
Methods
Our single institution's perioperative data was retrospectively collected. The primary outcome was post-operative LOS > 14 days. Longitudinal and circumferential STE deformation measures were analyzed on echocardiograms obtained during pre-operative catheterization. Patient-specific, echocardiographic, and catheterization data were included in multi-variable logistic regression. Receiver operating characteristic area under the curves (AUC) were analyzed.
Results
From 2007-2014, 135 who underwent TCPC could be included in the analysis. Median LOS was 11 (IQR 9-14) days. PVR (p<0.01) and circumferential strain rate (CSR) (p<0.01) were the only variables independently associated with LOS > 14 days. For every 0.1 s−1 CSR increased, there was a 20% increased odds of prolonged LOS. The AUC for CSR was 0.70. The AUC for PVR and EDP combined was 0.68. The AUC for PVR, EDP, and CSR combined was 0.73.
Conclusion
Pre-operative CSR is independently associated with LOS > 14 days and improves pre-operative risk stratification in patients undergoing TCPC.
Keywords: Single ventricle, Speckle-tracking echocardiography
Introduction
The current management strategy for children with single ventricle physiology is staged surgical palliation, ultimately leading to total cavopulmonary connection (TCPC) circulation. This circulation requires blood to flow passively from the cavae directly through the pulmonary arteries to the heart. The presence of ventricular dysfunction in the systemic ventricle often translates to the development of heart failure and resultant inefficiency within the TCPC pathway, which increases the risk for significant post-operative morbidity, early surgical palliation failure, and death.1-3
Pre-operative risk assessment prior to TCPC often includes invasively derived indices of pulmonary vascular resistance and end-diastolic pressure in the catheterization laboratory. Patients with significantly abnormal pulmonary vascular resistance or end-diastolic pressure do not undergo TCPC to avoid the morbidity and mortality associated with the resultant suboptimal hemodynamics after the operation. Even so, a subset of patients continue to experience significant morbidity, including prolonged hospital length of stay, after TCPC. Identifying these at risk patients has been difficult. Anecdotal evidence suggests those with poor ventricular function experience prolonged length of stay after TCPC. However, including ventricular function in the pre-operative risk assessment has been challenging as the echocardiographic assessment of ventricular function in single ventricle physiology is frequently qualitative with poor reproducibility.4 The quantitative assessment of single ventricular function is difficult due to complex and heterogenous ventricular geometry.5 Due to these challenges, a number of studies in the modern era have not included echocardiographic measures of ventricular function in their analyses of pre-operative risk assessment.6, 7 When included in risk models, qualitative echocardiographic measures have shown no value in predicting length of stay after TCPC.8
Speckle-tracking echocardiography (STE) is an attractive tool for use in the assessment of ventricular function in single ventricle physiology due to its angle independence, geometry independence, and reproducibility.9, 10 It has been shown to be more sensitive in detecting changes in ventricular function in children and adults with heart disease than more conventional measures, such as ejection fraction.11-13 However, no studies investigating the clinical usefulness of STE in predicting post-TCPC outcomes in single ventricle patients have been performed. The aims of this study were to: 1) investigate the association between pre-operative measures of myocardial deformation and post-operative length of stay (LOS) after TCPC and 2) determine if these STE measures of ventricular function improve risk stratification prior to TCPC over conventional risk factors obtained during invasive cardiac catheterization.
Methods
This was a retrospective analysis of patients with single ventricle physiology who underwent TCPC at the Medical University of South Carolina from 2007-2014. In order to standardize echocardiographic analysis, patients without a clearly dominant ventricle were excluded. Pre-operative catheterization data were abstracted from the patient record. Perioperative data included those variables which were reported to the Society of Thoracic Surgeon's database (all variables listed in Table 1). The primary outcome was post-operative LOS > 14 days which represented the top quartile. This study was reviewed and approved by the Medical University of South Carolina's Institutional Review Board.
Table 1.
Demographic, echocardiographic, catheterization, and perioperative data in single ventricle patients with length of stay ≤ 14 days vs. > 14 days
| LOS ≤ 14 days (n = 102) | LOS > 14 days (n = 33) | p-value | |
|---|---|---|---|
| Age (years) | 4.0 (3.2, 4.6) | 4.5 (3.6, 5.7) | 0.08 |
| Male, n (%) | 56 (55%) | 21 (63%) | 0.51 |
| Height (cm) | 98 (94, 103) | 101 (94, 110) | 0.23 |
| Weight (kg) | 15.0 (14.1, 16.2) | 15.2 (13.5, 16.8) | 0.98 |
| SBP (mm Hg) | 89 ± 13 | 82 ± 10 | 0.01 |
| DBP (mm Hg) | 45 (40, 50) | 42 (40, 47) | 0.49 |
| CPB time (minutes) | 109 (91, 142) | 118 (96, 151) | 0.61 |
| End diastolic pressure (mm Hg) | 7 (6, 9) | 8 (7, 9) | 0.03 |
| Transpulmonary gradient (mm Hg) | 4 (3, 5) | 4 (4, 6) | 0.41 |
| Rp (Wood units) | 1.6 (1.2, 2.0) | 2.0 (1.4, 2.3) | 0.07 |
| A-V O2 saturation difference (%) | 17 (14, 20) | 17 (15, 21) | 0.49 |
| Right ventricular dominance, n (%) | 60 (59%) | 23 (70%) | 0.28 |
| Presence of aorto-pulmonary collaterals, n (%) | 0.80 | ||
| None | 22 (21%) | 5 (15%) | |
| Small | 66 (65%) | 23 (70%) | |
| Large, coiled | 14 (14%) | 5 (15%) | |
| Atrioventricular valve regurgitation grade, n (%) | 0.04 | ||
| None - Trivial | 18 (17%) | 1 (3%) | |
| Mild | 75 (74%) | 25 (76%) | |
| Moderate | 8 (8%) | 6 (18%) | |
| Severe | 1 (1%) | 1 (3%) | |
| Fractional area change (%) | 24.1 (18.4, 31.1) | 22.1 (14.6, 29.4) | 0.28 |
| SAPSE (cm) | 0.60 (0.40, 0.80) | 0.60 (0.43, 0.78) | 0.89 |
| Ejection Fraction (%) | 43.4 ± 7.7 | 41.1 ± 10.6 | 0.18 |
| Longitudinal strain (%) | −15.4 ± 3.2 | −14.7 ± 4.4 | 0.97 |
| Longitudinal strain rate (s−1) | −1.08 (−1.24, −0.90) | −1.07 (−1.30, −0.75) | 0.78 |
| Longitudinal EDSR (s−1) | 1.21 (1.01, 1.45) | 1.17 (0.89, 1.49) | 0.50 |
| Circumferential strain (%) | −13.8 (−16.3, −11.4) | −11.9 (−14.7, −9.8) | 0.04 |
| Circumferential strain rate (s−1) | −1.05 (−1.27, −0.89) | −0.97 (−1.14, −0.61) | 0.02 |
| Circumferential EDSR (s−1) | 1.06 (0.84, 1.29) | 0.72 (0.65, 1.21) | 0.05 |
Results reported as mean ± standard deviation or median (interquartile range). A-V = arteriovenous, CBP = cardiopulmonary bypass, DBP = diastolic blood pressure, EDSR = early diastolic strain rate, LOS = length of stay, Rp = pulmonary vascular resistance, SAPSE = single ventricle atrioventricular valve systolic plane excursion, SBP = systolic blood pressure.
Echocardiographic Analysis
All echocardiographic studies were performed using a Phillips 7500 or IE33 ultrasound system (Andover, MA). All studies were performed under general anesthesia immediately prior to cardiac catheterization as is the institutional routine. Echocardiograms were stored in Digital Imaging and Communications in Medicine format at a frame rate of 30 frames/sec. Echocardiograms were analyzed retrospectively by a single blinded reviewer.
Conventional parameters for assessment of single ventricular function from the apical 4-chamber view included 1) 2D single ventricle annular plane systolic excursion measured as the difference in length from the lateral free wall of the dominant ventricle at the level of the atrioventricular valve to the apex in diastole vs. systole and 2) 2D fractional area change (FAC) calculated as (end-diastolic area - end-systolic area)/end-diastolic area of the dominant ventricle. Qualitative assessments of atrioventricular valve regurgitation and ventricular function were also recorded.
Speckle-tracking echocardiography was performed retrospectively on images stored for offline analysis using vendor independent software (Cardiac Performance Analysis v. 3.0; Tomtec, Hamden, CT). The endocardial border was manually traced and tracking was automatically performed by the software. Segments with inadequate tracking were excluded from the analysis. Global measures of longitudinal or circumferential deformation were excluded if > 2 segments displayed inadequate tracking. Six segments from the apical 4 chamber view were averaged to measure longitudinal strain, strain rate, and early diastolic strain rate. From this view, the software automatically calculates ejection fraction using single-plane Simpson's method. Six segments from the parasternal short axis view below the level of the atrioventricular valve(s) at the mid-ventricular level were averaged to calculate circumferential strain, strain rate, and early diastolic strain rate. A more negative systolic strain or strain rate indicates better ventricular function.
Statistical Analysis
The distribution of data as parametric or non-parametric was assessed using the Shapiro-Wilk test. Differences between patients with and without prolonged LOS were assessed using independent t-tests or Mann Whitney U tests, as appropriate for continuous variables and Chi-square test or Fisher's Exact test for categorical variables. Univariate logistic regression was performed to assess the relationship between independent variables and post-operative LOS > 14 days. Multivariable regression was then performed including independent variables that displayed a p-value of < 0.20 upon univariable analysis. Multivariable logistic regression was performed using stepwise elimination excluding independent variables with a p-value > 0.10 or those that did not significantly improve the explanatory power of the model (improvement in the Nagelkerke R2 value by 0.03 or more). Receiver operating characteristic curves and associated c-statistic were calculated to determine the discriminatory power of independent variables in predicting the primary outcome. Missing data in the regression analysis was imputed using the chained equations method over 5 imputations and pooled results are reported. This method operates under the assumption that given the variables used in the imputation procedure, the missing data are missing at random. In the procedure a series of regression models are run whereby each variable with missing data is modeled conditional upon the other variables in the data. This means that each variable can be modeled according to its distribution, with, for example, binary variables modeled using logistic regression and continuous variables modeled using linear regression. The analyses of multiply imputed data take into account the uncertainty in the imputations and yield accurate standard errors.14 Interobserver variability in speckle-tracking measures of myocardial deformation were assessed using intraclass correlation coefficients (ICC) of absolute agreement in 20% of studies. A p-value of < 0.05 was considered statistically significant. All statistics were performed using IBM SPSS v. 23 (New York, NY).
Results
From 2007-2014, 141 patients underwent TCPC. Of those, six were excluded as ventricular dominance could not be clearly identified. All remaining 135 patients were included in the analysis. Extra-cardiac conduits were performed in 123 patients (91%). Creation of a fenestration between the TCPC and the atrium was performed in 120 patients (89%). In general, fenestrations were not created if patients had evidence of pulmonary arteriovenous malformations on pre-operative catheterization. Right ventricular dominance was present in 83 (61%). The median LOS in the entire cohort was 11 days (IQR 9, 14). Thirty-three patients (24%) had a LOS > 14 days. Thirty-day mortality was 0%. Four patients (3%) were intubated > 1 day. Subject demographics, pre-operative echocardiographic and catheterization data, peri-operative data, and comparisons between patients with LOS ≤ 14 days and > 14 days can be found in Table 1.
There were no differences in LOS between patients of left vs. right dominant ventricular morphology [10 (IQR 9-12) days vs. 11 (IQR 9-15) days respectively, p = 0.41]. There were no differences in demographic, echocardiographic, catheterization, or peri-operative data in those with left vs. right ventricular dominant morphology (Table 2) with the exception of increased severity of atrioventricular valve regurgitation in those with right ventricular morphology (p < 0.01).
Table 2.
Demographic, echocardiographic, catheterization, and perioperative data in single ventricle patients with left vs. right ventricular dominance
| LV-dominant (n = 52) | RV-dominant (n = 83) | p-value | |
|---|---|---|---|
| Age (years) | 4.0 (3.3, 4.7) | 4.0 (3.3, 4.6) | 0.69 |
| Male, n (%) | 27 (52%) | 51 (61%) | 0.28 |
| Length of stay (days) | 10 (9, 12) | 11 (9, 15) | 0.41 |
| End diastolic pressure (mm Hg) | 8 (6, 10) | 7 (6, 9) | 0.50 |
| Rp (Wood units) | 1.6 (1.3, 2.1) | 1.8 (1.4, 2.1) | 0.48 |
| Atrioventricular valve regurgitation grade, n (%) | < 0.01 | ||
| None - Trivial | 38 (73%) | 40 (48%) | |
| Mild | 13 (25%) | 28 (34%) | |
| Moderate | 1 (2%) | 13 (15%) | |
| Severe | 0 (0%) | 2 (3%) | |
| Fractional area change (%) | 22.9 (18.3, 29.8) | 25.9 (19.6, 30.6) | 0.57 |
| SAPSE (cm) | 0.60 (0.40, 0.80) | 0.60 (0.40, 0.80) | 0.70 |
| Ejection Fraction (%) | 42.1 ± 6.9 | 42.5 ± 9.1 | 0.86 |
| Longitudinal strain (%) | −15.2 ± 3.4 | −15.4 ± 3.6 | 0.67 |
| Longitudinal strain rate (s−1) | −1.04 (−1.17, −0.83) | −1.04 (−1.25, −0.86) | 0.85 |
| Longitudinal EDSR (s−1) | 1.11 (0.96, 1.32) | 1.22 (0.93, 1.44) | 0.34 |
| Circumferential strain (%) | −14.7 (−17.1, −11.3) | −13.6 (−16.0, −11.1) | 0.39 |
| Circumferential strain rate (s−1) | −1.02 (−1.25, −0.86) | −0.97 (−1.23, −0.81) | 0.60 |
| Circumferential EDSR (s−1) | 1.13 (0.90, 1.30) | 1.05 (0.82, 1.32) | 0.44 |
Results reported as mean ± standard deviation or median (interquartile range). EDSR = early diastolic strain rate, Rp = pulmonary vascular resistance, SAPSE = single ventricle atrioventricular valve systolic plane excursion.
Longitudinal deformation parameters could be measured in 130 patients (96%) of patients. The four patients without longitudinal parameters performed had an incomplete view of the ventricular apex, and were all systemic right ventricles. Circumferential deformation parameters could be measured in 113 (84%) of patients. The majority that did not have circumferential deformation parameters (n = 20) performed were secondary to an absence of the parasternal short axis view through the mid ventricle. The other two who did not have circumferential deformation parameters measured were secondary to poor echocardiographic windows precluding analysis. The interobserver ICC for longitudinal strain, strain rate, and early diastolic strain rate were r = 0.91, r = 0.84, and r = 0.79, respectively. The interobserver ICC for circumferential strain, strain rate, and early diastolic strain rate were r = 0.86, r = 0.76, and r = 0.79, respectively. All p-values were < 0.01 for above ICCs.
Predictors of Length of Stay
Univariable logistic regression showed significant relationships between LOS > 14 days and circumferential strain rate (OR 6.2, p = 0.02), pulmonary vascular resistance (OR 2.0, p = 0.02), and systolic blood pressure (OR 0.96, p = 0.02). Covariables not statistically significantly associated with LOS > 14 days, but also included in the multivariable analysis (p ≤ 0.20) included age, year of TCPC, diastolic blood pressure, end-diastolic pressure, degree of atrioventricular valve regurgitation, cardiopulmonary bypass time, subjective assessment of systolic function, ejection fraction, circumferential strain, and circumferential early diastolic strain rate. Variables not included in the multivariable regression (p > 0.20) included weight, sex, ventricular dominance, trans-pulmonary gradient, creation of TCPC fenestration, presence of aorto-pulmonary collaterals, arterio-venous oxygen saturation difference, longitudinal strain, longitudinal strain rate, and longitudinal early diastolic strain rate.
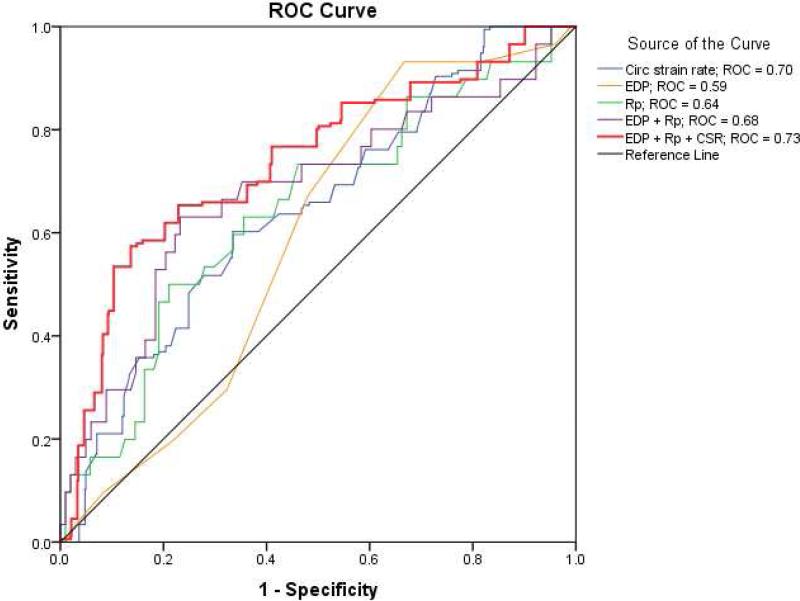
Upon multivariable logistic regression, only circumferential strain rate [B = 1.78, OR 6.3 (95% CI 1.4-28.2), p = 0.02] and pulmonary vascular resistance [B = 0.66, OR 2.0 (95% CI 1.1-3.7), p = 0.02] displayed a statistically significant relationship with LOS > 14 days. For every 0.1 s−1 circumferential strain rate increased, there was a 20% increased odds of a patient having prolonged LOS. Receiver operating characteristic curve analysis was performed on these two variables, as well as end-diastolic pressure as it is used clinically for risk stratification purposes. Receiver operating characteristic curve analysis showed an area under the curve for circumferential strain rate of 0.70, p = < 0.01, pulmonary vascular resistance of 0.64, p = 0.02, and end-diastolic pressure of 0.59, p = 0.01. A combined measure of end-diastolic pressure and pulmonary vascular resistance showed an area under the curve of 0.68, p < 0.01. A combined measure of circumferential strain rate, end-diastolic pressure, and pulmonary vascular resistance showed an area under the curve of 0.73, p < 0.01 (Figure 2). A circumferential strain rate of > −1.0 s−1 would have a 72% sensitivity and 60% specificity to predict a LOS > 14 days. No patients with a circumferential strain rate < −1.5 s−1 (n = 15) had a LOS > 14 days.
Figure 2.
Independent variable receiver-operating characteristic curves for length of stay > 14 days. CSR = circumferential strain rate. EDP = end-diastolic pressure; Rp = pulmonary vascular resistance.
Discussion
This is the first study to show that pre-operative speckle-tracking echocardiographic measures of deformation are associated with LOS after TCPC.
Predictors of length of stay
There was no relationship between conventional echocardiographic measures of ventricular function and LOS after TCPC in the current study. This is in line with previous single center and multi-institutional studies.8, 15-17 Speckle-tracking measures may give us better insight into post-operative outcomes because these measures of myocardial deformation are influenced more highly by contractility than are conventional measures.18 The relationship between speckle-tracking derived strain rate and contractility has indeed been confirmed in children with single ventricle physiology.19 Evaluating patients’ pre-operative contractile status is likely useful in predicting their hemodynamic response to TCPC - which includes a cardiopulmonary bypass event and significant volume unloading of a single ventricle. In our study, patients with a more negative circumferential strain rate (greater deformation rate) appeared to tolerate the hemodynamic insults associated with TCPC more favorably than those with a less negative circumferential strain rate.
We found that pulmonary vascular resistance was associated with prolonged LOS. This was expected as elevated pulmonary artery pressures after TCPC lead to elevated central venous pressure, increased chest tube drainage, and clinical right heart failure.6, 20, 21 In contrast to a previous multi-center study,8 we found no relationship between atrioventricular valve regurgitation and length of stay in our analysis. The mechanism of atrioventricular valve regurgitation is multifactorial in single ventricle patients, including, but not limited to, annular dilation from volume overload and leaflet tethering from papillary muscle displacement. Importantly, abnormal ventricular function contributes to atrioventricular valve dysfunction.22 By appropriately accounting for the confounding relationship between ventricular function and atrioventricular valve regurgitation in our analysis, we may have had inadequate power to detect the association between atrioventricular valve regurgitation and LOS in our analysis.
Differences between ventricular morphologies
We found no differences in LOS after TCPC between patients with right vs. left dominant ventricular morphologies. This is congruent with a number of previous studies.7, 16, 17 Interestingly, there were no differences in ventricular function as measured conventionally, or by speckle-tracking echocardiography, between groups. The adaptation of the systemic right ventricle from a longitudinal-dominant contraction pattern to a circumferential dominant contraction pattern has been described to occur in single ventricle patients after the first stage of palliation.23, 24 This study has shown that by the time of TCPC, there are no significant differences in contraction patterns or overall systolic function between the groups, similar to at least one previous study assessing only longitudinal function.25 These similarities may contribute to the similarities seen in long-term outcomes between these groups in the modern era.26
Clinical feasibility and implications
Performing speckle-tracking echocardiography in patients with single ventricle physiology was quite feasible in this retrospective study. Observer variability was also acceptable. Other studies investigating single ventricle patients are in agreement with these findings.10, 27, 28 The use of speckle-tracking echocardiography is becoming routine in many pediatric echocardiography laboratories.29 Incorporating speckle-tracking echocardiography into the pre-operative assessment prior to TCPC appears quite feasible in clinical practice and may improve the identification of high risk patients. For example, it seems that patients with a circumferential strain rate < −1.5 s−1 are at very low risk for a prolonged LOS and those with circumferential strain rate > −1.0 s−1 can be considered high risk. Once identified, the effect of optimizing pre-operative heart failure regimens on post-operative outcomes can be investigated.
The ability to reliably quantify single ventricular function by speckle-tracking echocardiography will allow not only allow clinicians to assess the contribution of ventricular function to short term outcomes, as performed in the current study, but also to assess its association with medium and long-term outcomes more accurately. The ability to detect modifiable risk factors for heart failure will become more feasible. Finally, as new technologies and techniques are developed, we will be able to accurately determine the effects of medical, interventional, and surgical procedures on single ventricular function.
Limitations
This study was limited by its retrospective nature, therefore a causal association between decreased ventricular function and LOS could not be determined. LOS is affected by many factors (heterotaxy, prior types of palliation, genetic syndrome, etc) that could not be accounted for in this analysis and may have confounded our results. We could not feasibly collect secondary outcome data, such as chest tube drainage days or presence of chylous drainage which are highly collinear with LOS, due to change from paper to electronic medical records in the middle of the study period. Patients who were excluded from undergoing TCPC were not included in this analysis – the ability to use STE to identify these patients cannot be inferred from the current study. All studies were performed under general anesthesia which limits the generalizability of our findings in institutions where pre-operative echocardiography is performed under different conditions. The duration of the study period was somewhat long, however, no significant changes to post-operative management was made in that time period. It was further limited by lack of a standardized imaging protocol on all pre-operative patients leading to an inability to perform speckle-tracking on some patients. In addition, echocardiograms were taken from the clinical server where they are stored in compressed DICOM format at 30 frames per second. Such conditions may lead to underestimation of strain rates.30 Our sample size was somewhat small in the setting of assessing multiple independent variables in the regression analysis. This may have led to overfitting of the data. In addition, cutoff values derived from ROC analysis in this study were not validated in a separate cohort. Our results should be verified in an independent cohort to ensure reproducibility of our findings.
Conclusions
Pre-operative circumferential strain rate is independently associated with LOS > 14 days after TCPC in patients with single ventricle physiology. The addition of circumferential strain rate to standard hemodynamic measurements during the pre-TCPC assessment improves pre-operative risk stratification in identifying high risk patients. The quantitative assessment of ventricular function in single ventricle patients by speckle-tracking echocardiography appears to be clinically useful prior to TCPC.
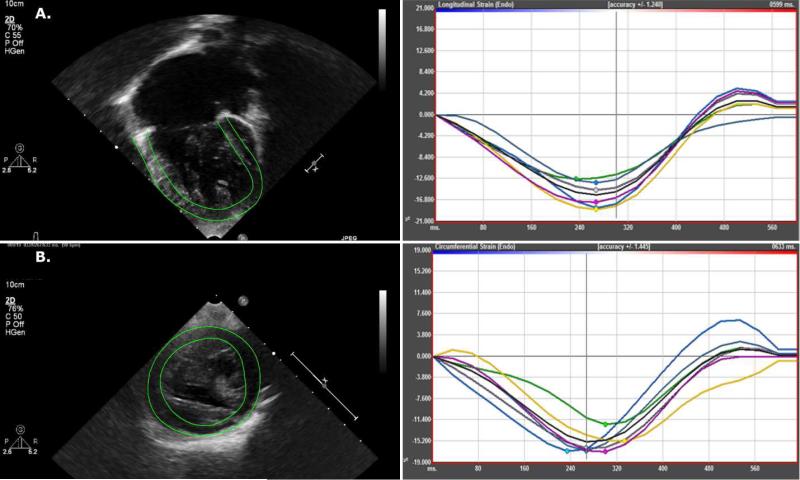
Figure 1.
Representative speckle-tracking analysis tracings of resultant deformation curves in a child with right ventricular dominant single ventricle physiology. A. Tracing of the right ventricle from the apical 4 chamber view and longitudinal strain deformation curve. B. Tracing of the right ventricle from the parasternal short axis view and circumferential strain deformation curve.
Highlights.
There has been a limited role of echocardiography in risk stratifying patients undergoing total cavopulmonary connection
Aim: investigate the association between measures of myocardial deformation and post-operative length of stay after total cavopulmonary connection
Pre-operative circumferential strain rate was associated with length of stay > 14 days
Circumferential strain rate improved pre-operative risk stratification in these patients
Acknowledgments
Funding Sources
Dr. Park was supported by NIH/NHLBI grant T32 HL07710. Dr. Chowdhury is supported by American Heart Association Mentored Clinical and Population Research Program Grant 15MCPRP25820008.
Abbreviations
- CSR
Circumferential strain rate
- FAC
fractional area change
- ICC
intraclass correlation coefficients
- LOS
length of stay
- STE
speckle-tracking echocardiography
- TCPC
total cavopulmonary connection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Williams IA, Sleeper LA, Colan SD, Lu M, Stephenson EA, Newburger JW, et al. Functional state following the Fontan procedure. Cardiol Young. 2009;19:320–30. doi: 10.1017/S1047951109990382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Border WL, Syed AU, Michelfelder EC, Khoury P, Uzark KC, Manning PB, et al. Impaired systemic ventricular relaxation affects postoperative short-term outcome in Fontan patients. J Thorac Cardiovasc Surg. 2003;126:1760–4. doi: 10.1016/j.jtcvs.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Mair DD, Hagler DJ, Puga FJ, Schaff HV, Danielson GK. Fontan operation in 176 patients with tricuspid atresia. Results and a proposed new index for patient selection. Circulation. 1990;82:IV164–9. [PubMed] [Google Scholar]
- 4.Bellsham-Revell HR, Simpson JM, Miller OI, Bell AJ. Subjective evaluation of right ventricular systolic function in hypoplastic left heart syndrome: how accurate is it? J Am Soc Echocardiogr. 2013;26:52–6. doi: 10.1016/j.echo.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margossian R, Schwartz ML, Prakash A, Wruck L, Colan SD, Atz AM, et al. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study). Am J Cardiol. 2009;104:419–28. doi: 10.1016/j.amjcard.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers LS, Glatz AC, Ravishankar C, Spray TL, Nicolson SC, Rychik J, et al. 18 Years of the Fontan Operation at a Single Institution: Results From 771 Consecutive Patients. J Am Coll Cardiol. 2012;60:1018–1025. doi: 10.1016/j.jacc.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien JE, Jr., Marshall JA, Young AR, Handley KM, Lofland GK. The nonfenestrated extracardiac Fontan procedure: a cohort of 145 patients. Ann Thorac Surg. 2010;89:1815–20. doi: 10.1016/j.athoracsur.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 8.Ravishankar C, Gerstenberger E, Sleeper LA, Atz AM, Affolter JT, Bradley TJ, et al. Factors affecting Fontan length of stay: Results from the Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2016;151:669–675. e1. doi: 10.1016/j.jtcvs.2015.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoo NS, Tham EB, Kantor PF. Newer imaging modalities in the assessment of heart function in single ventricle hearts. Can J Cardiol. 2013;29:886–9. doi: 10.1016/j.cjca.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Singh GK, Cupps B, Pasque M, Woodard PK, Holland MR, Ludomirsky A. Accuracy and reproducibility of strain by speckle tracking in pediatric subjects with normal heart and single ventricular physiology: a two-dimensional speckle-tracking echocardiography and magnetic resonance imaging correlative study. J Am Soc Echocardiogr. 2010;23:1143–52. doi: 10.1016/j.echo.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toro-Salazar OH, Ferranti J, Lorenzoni R, Walling S, Mazur W, Raman SV, et al. Feasibility of Echocardiographic Techniques to Detect Subclinical Cancer Therapeutics-Related Cardiac Dysfunction among High-Dose Patients When Compared with Cardiac Magnetic Resonance Imaging. J Am Soc Echocardiogr. 2016;29:119–31. doi: 10.1016/j.echo.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 12.den Boer SL, du Marchie Sarvaas GJ, Klitsie LM, van Iperen GG, Tanke RB, Helbing WA, et al. Longitudinal Strain as Risk Factor for Outcome in Pediatric Dilated Cardiomyopathy. JACC Cardiovasc Imaging. 2015 doi: 10.1016/j.jcmg.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong GT, Joshi VM, Ness KK, Marwick TH, Zhang N, Srivastava D, et al. Comprehensive Echocardiographic Detection of Treatment-Related Cardiac Dysfunction in Adult Survivors of Childhood Cancer: Results From the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65:2511–22. doi: 10.1016/j.jacc.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple Imputation by Chained Equations: What is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tweddell JS, Nersesian M, Mussatto KA, Nugent M, Simpson P, Mitchell ME, et al. Fontan Palliation in the Modern Era: Factors Impacting Mortality and Morbidity. Ann Thorac Surg. 2009;88:1291–1299. doi: 10.1016/j.athoracsur.2009.05.076. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch JC, Goldberg C, Bove EL, Salehian S, Lee T, Ohye RG, et al. Fontan operation in the current era: a 15-year single institution experience. Ann Surg. 2008;248:402–10. doi: 10.1097/SLA.0b013e3181858286. [DOI] [PubMed] [Google Scholar]
- 17.Salvin JW, Scheurer MA, Laussen PC, Mayer JE, Jr., Del Nido PJ, Pigula FA, et al. Factors associated with prolonged recovery after the fontan operation. Circulation. 2008;118:S171–6. doi: 10.1161/CIRCULATIONAHA.107.750596. [DOI] [PubMed] [Google Scholar]
- 18.Yotti R, Bermejo J, Benito Y, Sanz-Ruiz R, Ripoll C, Martinez-Legazpi P, et al. Validation of noninvasive indices of global systolic function in patients with normal and abnormal loading conditions: a simultaneous echocardiography pressure-volume catheterization study. Circ Cardiovasc Imaging. 2014;7:164–72. doi: 10.1161/CIRCIMAGING.113.000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlangen J, Petko C, Hansen JH, Michel M, Hart C, Uebing A, et al. Two-dimensional global longitudinal strain rate is a preload independent index of systemic right ventricular contractility in hypoplastic left heart syndrome patients after fontan operation. Circ Cardiovasc Imaging. 2014;7:880–6. doi: 10.1161/CIRCIMAGING.114.002110. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki J, Dykes JC, Sosa LJ, Salvaggio JL, Tablante MD, Ojito J, et al. Risk Factors for Longer Hospital Stay Following the Fontan Operation. Pediatr Crit Care Med. 2016;17:411–9. doi: 10.1097/PCC.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 21.Mendoza A, Albert L, Ruiz E, Boni L, Ramos V, Velasco JM, et al. Fontan Operation. Hemodynamic Factors Associated With Postoperative Outcomes. Rev Esp Cardiol (Engl Ed) 2012;65:356–362. doi: 10.1016/j.recesp.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Kutty S, Colen T, Thompson RB, Tham E, Li L, Vijarnsorn C, et al. Tricuspid regurgitation in hypoplastic left heart syndrome: mechanistic insights from 3-dimensional echocardiography and relationship with outcomes. Circ Cardiovasc Imaging. 2014;7:765–72. doi: 10.1161/CIRCIMAGING.113.001161. [DOI] [PubMed] [Google Scholar]
- 23.Khoo NS, Smallhorn JF, Kaneko S, Myers K, Kutty S, Tham EB. Novel insights into RV adaptation and function in hypoplastic left heart syndrome between the first 2 stages of surgical palliation. JACC Cardiovasc Imaging. 2011;4:128–37. doi: 10.1016/j.jcmg.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Tham EB, Smallhorn JF, Kaneko S, Valiani S, Myers KA, Colen TM, et al. Insights into the evolution of myocardial dysfunction in the functionally single right ventricle between staged palliations using speckle-tracking echocardiography. J Am Soc Echocardiogr. 2014;27:314–22. doi: 10.1016/j.echo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Petko C, Hansen JH, Scheewe J, Rickers C, Kramer HH. Comparison of longitudinal myocardial deformation and dyssynchrony in children with left and right ventricular morphology after the Fontan operation using two-dimensional speckle tracking. Congenit Heart Dis. 2012;7:16–23. doi: 10.1111/j.1747-0803.2011.00607.x. [DOI] [PubMed] [Google Scholar]
- 26.Atz AM, Zak V, Mahony L, Uzark K, Shrader P, Gallagher D, et al. Survival data and predictors of functional outcome an average of 15 years after the Fontan procedure: the pediatric heart network Fontan cohort. Congenit Heart Dis. 2015;10:E30–42. doi: 10.1111/chd.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghelani SJ, Harrild DM, Gauvreau K, Geva T, Rathod RH. Echocardiography and magnetic resonance imaging based strain analysis of functional single ventricles: a study of intra- and inter-modality reproducibility. Int J Cardiovasc Imaging. 2016 doi: 10.1007/s10554-016-0882-4. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt R, Orwat S, Kempny A, Schuler P, Radke R, Kahr PC, et al. Value of speckle-tracking echocardiography and MRI-based feature tracking analysis in adult patients after Fontan-type palliation. Congenit Heart Dis. 2014;9:397–406. doi: 10.1111/chd.12156. [DOI] [PubMed] [Google Scholar]
- 29.Colquitt JL, Pignatelli RH. Strain Imaging: The Emergence of Speckle Tracking Echocardiography into Clinical Pediatric Cardiology. Congenit Heart Dis. 2016;11:199–207. doi: 10.1111/chd.12334. [DOI] [PubMed] [Google Scholar]
- 30.Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Jaeggi ET, Mertens L, et al. Assessment of myocardial deformation in children using Digital Imaging and Communications in Medicine (DICOM) data and vendor independent speckle tracking software. J Am Soc Echocardiogr. 2011;24:37–44. doi: 10.1016/j.echo.2010.09.018. [DOI] [PubMed] [Google Scholar]