Abstract
Periprosthetic shoulder infection (PSI) is rare but potentially devastating. The rate of PSI is increased in cases of revision procedures, reverse shoulder implants and co-morbidities. One specific type of PSI is the occurrence of low-grade infections caused by non-suppurative bacteria such as Propionibacterium acnes or Staphylococcus epidemermidis.
Success of treatment depends on micro-organism identification, appropriate surgical procedures and antibiotic administration efficiency. Post-operative early PSI can be treated with simple debridement, while chronic PSI requires a one- or two-stage revision procedure. Indication for one-time exchange is based on pre-operative identification of a causative agent. Resection arthroplasty remains an option for low-demand patients or recalcitrant infection.
Cite this article: EFORT Open Rev 2017;2:104-109. DOI: 10.1302/2058-5241.2.160023
Keywords: infection, arthroplasty, shoulder, Propionibacterium acnes, revision
Introduction
While more than 66 000 prosthetic shoulder procedures were performed in 2011 in the United States, the rate of post-operative infection seems to remain stable with 0.98% of cases.1-3 However, when infection occurs, this complication is always devastating with significant clinical and socioeconomic consequences.2 The rate is higher after revision surgery than after a primary procedure and reaches close to 5% in cases of reverse shoulder arthroplasty (RSA).4,5 Patients undergoing primary RSA are found to have a six times greater risk of infection compared with patients having primary unconstrained total shoulder arthroplasty.6 Arthroplasties for trauma are more at risk of infection than those from other causes.6 Comorbidities such as coagulopathy, renal failure, diabetes, lupus erythematosus, rheumatoid arthritis, intra-articular steroid injections and corticosteroid therapy increase the risk of periprosthetic shoulder infection (PSI).7 PSI is the major cause for revision within the first two post-operative years after an arthroplasty.8
The aim of this review is to investigate PSI from diagnosis to prevention and to report the main results of different therapeutic options.
Microbiology
The micro-organisms most commonly isolated in cases o f PSI are Staphylococcus (S.) aureus, S. epidermidis and Propionibacterium (P.) acnes.9 P. acnes is a gram-positive anaerobic bacillus, much more concentrated in the axilla than in the knee or hip.10 Different studies have identified a presence of P. acnes in the operative field during primary surgery, despite rigorous skin preparation and timely administered prophylactic antibiotics.11-13 It colonises the pilosebaceous follicles and there is a likelihood of finding it in the dermal layer, in male patients and after corticoid injection.12-14 Hudek et al14 identified a positive culture twofold greater for the anterolateral approach than for the deltopectoral approach. Surprisingly, it is even thought to be involved in the pathogenesis of glenohumeral osteoarthritis.11 It is often responsible for low-grade infection, with mild clinical symptoms, so that many classic clinical patterns do not strictly apply. The mean duration for culture incubation is between seven and > 21 days.
Prevention
Antibiotic prophylaxis is not specific to shoulder arthroplasty compared with other arthroplasties. Intravenous cephalosporine (2 g) administration is mandatory, given 30 minutes before the skin incision in many countries. However, some authors recommend a single 160 mg of gentamicin by intra-articular injection at the end of the procedure to reduce the risk of PSI.15
Saltzman et al16 have shown that pre-operative preparation of the surgical site with ‘ChloraPrep’ 2% (chlorhexidine gluconate and 70% isopropyl alcohol; ChloraPrep, Leawood, Kansas) was more effective than ‘DuraPrep’ (iodine povacrylex and 74% isopropyl alcohol; 3M, Minneapolis, Minnesota) and povidone-iodine (Purdue Pharma, Stamford, Connecticut) at eliminating overall bacteria, and that ChloraPrep and Duraprep were more effective than povidone-iodine regarding coagulase-negative Staphylococcus.
Hair removal is commonly performed before orthopaedic procedures and the use of razors is classically discouraged because micro-abrasions are created by shaving. However, removal of axillary hairs for shoulder surgery did not prove to have any effect on the cell-count of P. acnes before surgical preparation.17
Diagnosis
Classically, post-operative infections can be classified into acute infection (one to three months), subacute infection (four to 12 months) and late infection (> 12 months), depending on the time of diagnosis after the surgery.18 However, some authors advocate the distinguishing of early and late infection (cutoff at six weeks), so that there is a real chance to save the index prosthesis by avoiding any delay.19
One specific type of PSI is low-grade infections by non-suppurative bacteria such as P. acnes or S. epidemermidis.20,21 Diagnosis remains a challenge because P. acnes does not usually cause swelling, erythema, fever, purulent discharge or increasing level of biological parameters such as C-reactive protein (CRP), white blood cell count (WBC) and interleukin (IL)-6. Aspiration of synovial fluid is mandatory if a distinct fluid collection is identified (with positive serum levels giving positive findings of WBC > 3000/mm3 and > 80% for polymorphonuclear neutrophils). Also, deep biopsies that contact the prosthesis are needed.22-24 Performing five to six independent samples in order to optimise the sensitivity and specificity for the diagnosis of a PSI has been advocated. The cutoff for a definite diagnostic of infection should be three or more samples positive to the same micro-organism.24 The concern is that the growth duration from intra-operative samples is long for P. acnes, and every laboratory should be aware that they should not discard the culture samples early. However, early positive cultures seem to be more predictive of a true infection than a late growth culture, which can be a false-positive result.20
On the other hand, in the case of a virulent micro-organism, patients with PSI can also present classical symptoms including a painful shoulder with local and/or systemic physical signs. The blood tests reveal the same non-specific inflammatory markers that can be identified in a knee or hip prosthetic infection. Obviously, erythrocyte sedimentation, as well as CRP, have a poor sensitivity for the diagnosis of an infected arthroplasty in the post-operative setting, even if the CRP is normalised two weeks after an uneventful procedure.25
As proposed by a workgroup from the Musculoskeletal Infection Society focused on lower limb arthroplasty infections, the diagnosis should be based on a combination of biological and clinical criteria (Table 1).26
Table 1.
Criteria of Musculoskeletal Infection Society (MSIS) for retaining the prosthesis in periprosthetic infection26
| There is a sinus tract communicating with the prosthesis; or |
| A pathogen is isolated by culture from at least two separate tissue or fluid samples obtained from the affected prosthetic joint; or |
Four of the following criteria exist:
|
Treatment
Superficial wound infections are usually diagnosed in post-operative care and require local measures and antibiotic therapy. However, it is always necessary to suspect a deep infection and the patient should be treated as such until proven otherwise. In this potentially serious situation, success depends on early identification of micro-organisms, appropriate surgical procedures and efficient antibiotic administration. Different therapeutic options are available: debridement, simple resection arthroplasty, removal of the prosthesis and replacement with a cement spacer (spacer), single-stage revision, two-stage revision, arthrodesis, chronic antibiotic administration and even amputation. The outcomes of some of these options are described below.
Debridement
Debridement, irrigation and multiple deep samples may be proposed in cases of acute infection in order to save the prosthesis. Coste et al27 treated eight cases of acute infection with debridement and succeeded when it was performed within eight days after the diagnosis. They concluded that the earlier the debridement is done, the more effective it is in eradicating the infection. This procedure can be repeated, based on the patient’s response.28 Moreover, mobile parts of the prosthesis may be exchanged during the procedure especially in case of RSA (glenosphere, polyethylene liner) providing better access for debridement. Then, an appropriate antibiotics regime is required for a minimum of four weeks.21,28,29 However, the rate of success reported in the literature is only in the range of 50% to 95%.22,27-29
Cement spacer
An antibiotic-loaded cement spacer can be used either permanently or as the first step of a two-stage revision procedure. In this case, it maintains the space and soft-tissue tension for re-implantation and theoretically releases antibiotics to decrease the growth of microorganisms. Antibiotic mean concentration peak is reached at day 1 and dramatically decreases during the following seven days.30
Levy et al31 described a ‘functional cement spacer’ model, which is made of a hemi-arthroplasty coated with cement. In their series of 14 patients initially chosen for a two-stage procedure, nine did not undergo a prosthesis re-implantation because of satisfactory clinical outcomes. On the other hand, Verhelst et al32 did not prove any difference between patients with a cement spacer and resection arthroplasty regarding infection control and clinical outcomes. Complications of the cement-spacer such as breakage, glenoid erosion or dislocation have been reported.32
One-stage revision arthroplasty
Based on the experience of knee and hip infection management, a single-stage exchange is proposed as a reasonable option when the infecting micro-organism is satisfactiorily identified. The advantages are a reduced hospital stay, costs, period of antibiotic administration and the best clinical outcomes (Table 2).9,22,27,29,33-37
Table 2.
One-stage revision arthroplasty (RSA, reverse shoulder arthroplasty; HA, hemiarthroplasty; TSA, total shoulder arthroplasty; Bip, bipolar arthroplasty)
| Reference | n | Mean follow-up (yrs) |
Type of infected prosthesis | Rate of success (%) |
|---|---|---|---|---|
| Klatte et al33 | 35 | 4.7 | RSA, HA, Bip | 94 |
| Grosso et al37 | 17 | 3 | RSA, TSA, HA | 94.1 |
| Beekman et al36 | 11 | 2 | RSA | 91 |
| Ince et al34 | 9 | 5.8 | TSA, HA | 100 |
| Cuff et al35 | 7 | 3.6 | TSA, HA | 100 |
| Coste et al27 | 3 | 2.7 | TSA, HA | 100 |
| Jacquot et al21 | 5 | 3 | RSA | 100 |
Klatte et al33 reported the outcomes of the largest single-centre series of 35 patients with a mean follow-up of 4.7 years. The authors excised infected tissues, thoroughly irrigated the wound using pulsatile lavage with polyhexanide before re-implantation and delivered specific intravenous antibiotherapy for an average of two weeks post-operatively. The success rate was more than 90%. No recurrence was observed in the series of Coste et al,27 Ince et al34 and Cuff et al.35 The presence of a productive fistula seems not to be a contra-indication for many authors.33,36 Beekman et al36 performed a one-stage revision in 11 cases of RSAs, among which eight had a fistula, and achieved a success rate of 90%.
Two-stage revision arthroplasty
In a medically stable patient with a high demand, a two-stage revision procedure is generally accepted (Table 3).22,27,29,33,38-40 It is highly recommended when the micro-organism responsible for the infection is unknown. The first step consists of infection eradication after prosthetic removal: an antibiotic-loaded cement spacer is often implanted and general antibiotics are administrated, secondarily adapted to the micro-organism(s) identified. Antibiotics are generally continued for six to eight weeks. Markers such as CRP or IL-6 have been shown to be valuable to predict the eradication of infection and, so, the time of re-implantation.41,42 However, IL-6 seems to be normalised faster than CRP and allows earlier revision for better outcomes.41 An iterative irrigation and debridement could be proposed in case of persistent infection. For re-implantation, RSA has been gaining ground in recent years as the implant of choice. First, it allows a larger debridement at the first stage with less concern for soft-tissue preservation. Secondly, it offers the possibility of addressing the glenoid bone defect with or without bone graft. Shirwaiker et al43 reported that there is still uncertainty whether two-stage revision is superior to one-stage (Fig. 1).
Table 3.
Two-stage revision prosthesis (RSA, reverse shoulder arthroplasty; HA, hemiarthroplasty; TSA, total shoulder arthroplasty)
| Reference | n | Mean follow-up (yrs) | Type of infected prosthesis | Rate of success (%) |
|---|---|---|---|---|
| Strickland et al38 | 19 | 2.9 | HA, TSA | 63 |
| Romanò et al29 | 17 | 3.8 | RSA, HA | 100 |
| Sabesan et al40 | 17 | 3.8 | RSA, TSA, HA | 94 |
| Jacquot et al21 | 14 | 3 | RSA | 64 |
| Ortmaier et al39 | 12 | 6.1 | RSA | 75 |
| Coste et al27 | 10 | 2.6 | TSA, HA | 60 |
| Cuff et al35 | 10 | 3.6 | HA | 100 |
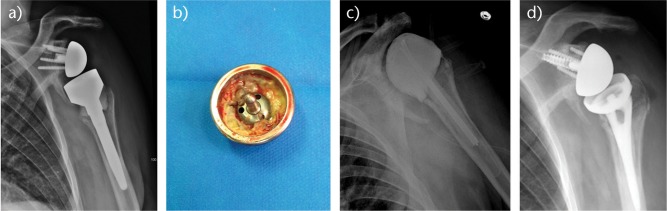
Fig. 1.
a) Radiograph of a 73-year-old man with a chronic periprosthetic shoulder infection of a reverse shoulder arthroplasty (RSA). b) A two-stage revision was decided with a cement spacer implantation for eight weeks. c) Propionibacterium acnes was identified on peri-operative samples taken from the back of the glenosphere. d) After four weeks free of antibiotics, a new RSA was implanted with a proximal humeral allograft.
Resection arthroplasty
Shoulder resection should remain a salvage procedure for frail or low-demand patients, and recalcitrant infection. It offers the option of a single definitive procedure for infection eradication (Fig. 2). It has been shown that functional results are poor, but pain relief is achieved in more than 50% of cases.9,44 Rispoli et al44 reported a mean active elevation of 70° at long-term follow-up after ‘anatomical’ shoulder arthroplasty removal. Verhelst et al32 demonstrated that preservation of the tuberosities is a predictive factor for better results, because it can avoid antero-superior subluxation of the humerus. In cases of RSA, Jacquot et al21 did not improve functional outcomes after removal of the implant and identified a high rate of post-operative complications. Bone loss and soft-tissue impairment after such constrained prostheses could partly explain these findings. Despite Jacquot21 and Coste’s27 studies, the literature reports a high rate of infection eradication, reaching more than 90% of cases.9,27,29,32,44,45
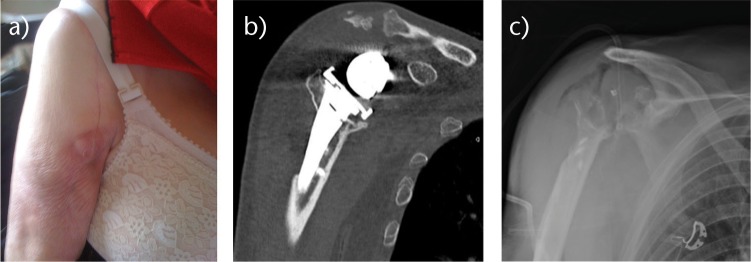
Fig. 2.
Radiographs (a and b) of an 86-year-old woman, with a loose implant secondary to chronic periprosthetic shoulder infection. c) Because of numbers of co-morbidities and huge bone loss on glenoid side, a simple resection arthroplasty was performed.
Conclusions
PSIs are a rare but remain a devastating complication in terms of functional outcomes. Acute infection (less than two months post-operatively) requires prosthetic washout as soon as possible, and the exchange of the mobile part of the implant. However, the important point in managing PSI is the high rate of low-grade infections that must be suspected in cases with abnormal clinical outcomes including pain and stiffness. It is often considered as sub-acute or chronic infection because of a ‘wait and see’ approach by the practitioner.46 In this situation, S. epidermidis or P. acnes are frequently involved and a simple debridement is too late. One-stage revision is possible if the micro-organism is identified pre-operatively, otherwise a two-stage procedure is recommended. Resection arthroplasty remains the option for low-demand patients. A multidisciplinary collaboration is nowadays recommended to optimise the antibiotic treatment and the surgical procedure.
Footnotes
ICMJE Conflict of interest statement: NB reports he is a consultant for Wright, Smith & Nephew and Stryker. PM reports that he receives personal fees from Wright, Smith & Nephew and Synthes. All other authors declare no conflict of interest.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg 2015;24:91-97. [DOI] [PubMed] [Google Scholar]
- 2. Padegimas EM, Maltenfort M, Ramsey ML, et al. Periprosthetic shoulder infection in the United States: incidence and economic burden. J Shoulder Elbow Surg 2015;24:741-746. [DOI] [PubMed] [Google Scholar]
- 3. Day JS, Lau E, Ong KL, et al. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg 2010;19:1115-1120. [DOI] [PubMed] [Google Scholar]
- 4. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011;20:146-157. [DOI] [PubMed] [Google Scholar]
- 5. Morris BJ, O’Connor DP, Torres D, et al. Risk factors for periprosthetic infection after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:161-166. [DOI] [PubMed] [Google Scholar]
- 6. Richards J, Inacio MC, Beckett M, et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res 2014;472:2809-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smucny M, Menendez ME, Ring D, Feeley BT, Zhang AL. Inpatient surgical site infection after shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:747-753. [DOI] [PubMed] [Google Scholar]
- 8. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res 2013;471:3672-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saltzman MD, Marecek GS, Edwards SL, Kalainov DM. Infection after shoulder surgery. J Am Acad Orthop Surg 2011;19:208-218. [DOI] [PubMed] [Google Scholar]
- 10. Patel A, Calfee RP, Plante M, Fischer SA, Green A. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg 2009;18:897-902. [DOI] [PubMed] [Google Scholar]
- 11. Levy O, Iyer S, Atoun E, et al. Propionibacterium acnes: an underestimated etiology in the pathogenesis of osteoarthritis? J Shoulder Elbow Surg 2013;22:505-511. [DOI] [PubMed] [Google Scholar]
- 12. Lee MJ, Pottinger PS, Butler-Wu S, et al. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg [Am] 2014;96:1447-1450. [DOI] [PubMed] [Google Scholar]
- 13. Maccioni CB, Woodbridge AB, Balestro JC, et al. Low rate of Propionibacterium acnes in arthritic shoulders undergoing primary total shoulder replacement surgery using a strict specimen collection technique. J Shoulder Elbow Surg 2015;24:1206-1211. [DOI] [PubMed] [Google Scholar]
- 14. Hudek R, Sommer F, Kerwat M, et al. Propionibacterium acnes in shoulder surgery: true infection, contamination, or commensal of the deep tissue? J Shoulder Elbow Surg 2014;23:1763-1771. [DOI] [PubMed] [Google Scholar]
- 15. Lovallo J, Helming J, Jafari SM, et al. Intraoperative intra-articular injection of gentamicin: will it decrease the risk of infection in total shoulder arthroplasty? J Shoulder Elbow Surg 2014;23:1272-1276. [DOI] [PubMed] [Google Scholar]
- 16. Saltzman MD, Nuber GW, Gryzlo SM, Marecek GS, Koh JL. Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg [Am] 2009;91-A:1949-1953. [DOI] [PubMed] [Google Scholar]
- 17. Marecek GS, Weatherford BM, Fuller EB, Saltzman MD. The effect of axillary hair on surgical antisepsis around the shoulder. J Shoulder Elbow Surg 2015;24:804-808. [DOI] [PubMed] [Google Scholar]
- 18. Franceschini V, Chillemi C. Periprosthetic shoulder infection. Open Orthop J 2013;7:243-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Härle A. Infection management in total hip replacement. Arch Orthop Trauma Surg 1989;108:63-71. [DOI] [PubMed] [Google Scholar]
- 20. Frangiamore SJ, Saleh A, Grosso MJ, et al. Early versus late culture growth of Propionibacterium acnes in revision shoulder arthroplasty. J Bone Joint Surg [Am] 2015;97:1149-1158. [DOI] [PubMed] [Google Scholar]
- 21. Jacquot A, Sirveaux F, Roche O, et al. Surgical management of the infected reversed shoulder arthroplasty: a French multicenter study of reoperation in 32 patients. J Shoulder Elbow Surg 2015;24:1713-1722. [DOI] [PubMed] [Google Scholar]
- 22. Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res 2001;382:206-216. [DOI] [PubMed] [Google Scholar]
- 23. Dilisio MF, Miller LR, Warner JJ, Higgins LD. Arthroscopic tissue culture for the evaluation of periprosthetic shoulder infection. J Bone Joint Surg [Am] 2014;96:1952-1958. [DOI] [PubMed] [Google Scholar]
- 24. Zhang AL, Feeley BT, Schwartz BS, Chung TT, Ma CB. Management of deep postoperative shoulder infections: is there a role for open biopsy during staged treatment? J Shoulder Elbow Surg 2015;24:e15-e20. [DOI] [PubMed] [Google Scholar]
- 25. Niskanen RO, Korkala O, Pammo H. Serum C-reactive protein levels after total hip and knee arthroplasty. J Bone Joint Surg [Br] 1996;78-B:431-433. [PubMed] [Google Scholar]
- 26. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 2011;469:2992-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coste JS, Reig S, Trojani C, et al. The management of infection in arthroplasty of the shoulder. J Bone Joint Surg [Br] 2004;86-B:65-69. [PubMed] [Google Scholar]
- 28. Duncan SF, Sperling JW. Treatment of primary isolated shoulder sepsis in the adult patient. Clin Orthop Relat Res 2008;466:1392-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Romanò CL, Borens O, Monti L, Meani E, Stuyck J. What treatment for periprosthetic shoulder infection? Results from a multicentre retrospective series. Int Orthop 2012;36:1011-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anagnostakos K, Wilmes P, Schmitt E, Kelm J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop 2009;80:193-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levy JC, Triplet J, Everding N. Use of a functional antibiotic spacer in treating infected shoulder arthroplasty. Orthopedics 2015;38:e512-e519. [DOI] [PubMed] [Google Scholar]
- 32. Verhelst L, Stuyck J, Bellemans J, Debeer P. Resection arthroplasty of the shoulder as a salvage procedure for deep shoulder infection: does the use of a cement spacer improve outcome? J Shoulder Elbow Surg 2011;20:1224-1233. [DOI] [PubMed] [Google Scholar]
- 33. Klatte TO, Junghans K, Al-Khateeb H, et al. Single-stage revision for peri-prosthetic shoulder infection: outcomes and results. Bone Joint J 2013;95-B:391-395. [DOI] [PubMed] [Google Scholar]
- 34. Ince A, Seemann K, Frommelt L, Katzer A, Loehr JF. One-stage exchange shoulder arthroplasty for peri-prosthetic infection. J Bone Joint Surg [Br] 2005;87-B:814-818. [DOI] [PubMed] [Google Scholar]
- 35. Cuff DJ, Virani NA, Levy J, et al. The treatment of deep shoulder infection and glenohumeral instability with debridement, reverse shoulder arthroplasty and postoperative antibiotics. J Bone Joint Surg [Br] 2008;90-B:336-342. [DOI] [PubMed] [Google Scholar]
- 36. Beekman PD, Katusic D, Berghs BM, Karelse A, De Wilde L. One-stage revision for patients with a chronically infected reverse total shoulder replacement. J Bone Joint Surg [Br] 2010;92-B:817-822. [DOI] [PubMed] [Google Scholar]
- 37. Grosso MJ, Sabesan VJ, Ho JC, Ricchetti ET, Iannotti JP. Reinfection rates after 1-stage revision shoulder arthroplasty for patients with unexpected positive intraoperative cultures. J Shoulder Elbow Surg 2012;21:754-758. [DOI] [PubMed] [Google Scholar]
- 38. Strickland JP, Sperling JW, Cofield RH. The results of two-stage re-implantation for infected shoulder replacement. J Bone Joint Surg [Br] 2008;90-B:460-465. [DOI] [PubMed] [Google Scholar]
- 39. Ortmaier R, Resch H, Hitzl W, et al. Treatment strategies for infection after reverse shoulder arthroplasty. Eur J Orthop Surg Traumatol 2014;24:723-731. [DOI] [PubMed] [Google Scholar]
- 40. Sabesan VJ, Ho JC, Kovacevic D, Iannotti JP. Two-stage reimplantation for treating prosthetic shoulder infections. Clin Orthop Relat Res 2011;469:2538-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coffey MJ, Ely EE, Crosby LA. Treatment of glenohumeral sepsis with a commercially produced antibiotic-impregnated cement spacer. J Shoulder Elbow Surg 2010;19:868-873. [DOI] [PubMed] [Google Scholar]
- 42. Villacis D, Merriman JA, Yalamanchili R, et al. Serum interleukin-6 as a marker of periprosthetic shoulder infection. J Bone Joint Surg [Am] 2014;96:41-45. [DOI] [PubMed] [Google Scholar]
- 43. Shirwaiker RA, Springer BD, Spangehl MJ, et al. A clinical perspective on musculoskeletal infection treatment strategies and challenges. J Am Acad Orthop Surg 2015;23:S44-S54. [DOI] [PubMed] [Google Scholar]
- 44. Rispoli DM, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Pain relief and functional results after resection arthroplasty of the shoulder. J Bone Joint Surg [Br] 2007;89-B:1184-1187. [DOI] [PubMed] [Google Scholar]
- 45. Weber P, Utzschneider S, Sadoghi P, et al. Management of the infected shoulder prosthesis: a retrospective analysis and review of the literature. Int Orthop 2011;35:365-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Atkins BL, Athanasou N, Deeks JJ, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group J Clin Microbiol 1998;36:2932-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]