Fig. (1).
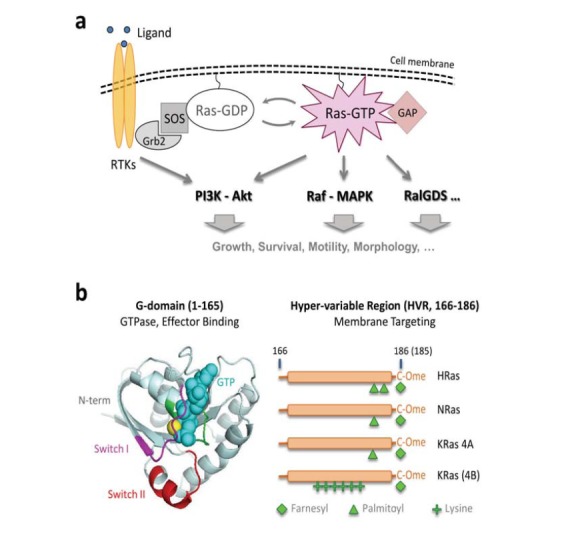
The Ras signaling pathways. (a) Ras resides on the inner leaflet of the membrane and transmits upstream signals such as those from ligand binding to receptor tyrosine kinases (RTKs). Activated RTKs recruit guanine nucleotide exchange factors (GEFs) such as SOS, which converts Ras-GDP into Ras-GTP; Ras-GTP then recruits and activates an array of effectors including PI3K, Raf, and RalGDS to execute specific cellular functions. The counteracting enzymes known as GTPase-activating proteins (GAPs) convert Ras-GTP back to Ras-GDP; (b) Mammalian cells ubiquitously express three Ras genes, H-, N-, and KRas, where KRas mRNA is alternatively spliced into the 4A and 4B forms. KRas 4B is commonly referred to as KRas. All four Ras isoforms have nearly identical G-domains comprised of a GTPase domain that binds and hydrolyzes GTP, and two switch regions I and II that undergo conformational change upon GTP loading to enable effector binding. The four isoforms differ in the last ~20 amino acids known as the hypervariable region (HVR), which contains a linker region (residues 166-186) and a CAAX (C=Cys; A=Aliphatic; X=any) box. After synthesis, Ras proteins are first farnesylated at the last Cys residue in the CAAX box. The AAX residues are subsequently removed and, depending on the Ras isoform (i.e., the sequence of the HVR), the protein can be further modified by different lipids. The post-translational modifications are critical to the correct membrane localization of Ras. HRas is dually palmitoylated, NRas and KRas 4A are mono palmitoylated, and KRas is not palmitoylated.
