Abstract
Background:
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a hereditary small artery disease caused by NOTCH3 gene mutation. We performed enhanced depth imaging optical coherence tomography (EDI-OCT) to evaluate the retinal vessel changes in CADASIL patients and assessed their consonance with brain magnetic resonance imaging (MRI) findings.
Methods:
Of 27 genetically confirmed patients and an equal number of controls were recruited at the Peking University First Hospital from January 2015 to August 2016. All patients underwent 7T-MRI of the brain. Fazekas score, number of small infarcts and microbleeds were evaluated. All patients and controls underwent EDI-OCT to measure subfoveal choroidal thickness (SFCT), inner and outer diameters as well as arterial and venous wall thickness, and arterial venous ratio of the inner (AVRin) and outer diameters (AVRout). The relation between retinal vessel changes and Fazekas scores, numbers of small infarcts, or microbleeds was analyzed. Paired t-test was used to compare the SFCT and retinal vessel measurement data between patients and controls. Spearman's correlation was used to investigate the correlation between retinal vessel changes and MRI lesions.
Results:
In CADASIL patients, mean SFCT (268.37 ± 46.50 μm) and mean arterial inner diameter (93.46 ± 9.70 μm) were significantly lower than that in controls (P < 0.001, P = 0.048, respectively). Mean arterial outer diameter (131.74 ± 10.87 μm), venous inner (128.99 ± 13.62 μm) and outer diameter (164.82 ± 14.77 μm), and mean arterial (19.13 ± 1.85 μm) and venous (17.91 ± 2.76 μm) wall thickness were significantly higher than that in controls (P = 0.023, P = 0.004, P < 0.001, P < 0.001, respectively). Arterial inner diameter (rs= −0.39, P = 0.044), AVRin (rs= −0.65, P < 0.001), and AVRout (rs= −0.56, P = 0.002) showed a negative correlation with the number of small infarcts. Venous inner diameter (rs = 0.46, P = 0.016) showed a positive correlation with the number of small infarcts. Venous inner diameter (rs = 0.59, P = 0.002), outer diameter (rs = 0.47, P = 0.017), showed a positive correlation with the number of cerebral microbleeds (CMBs). AVRin (rs= −0.52, P = 0.007) and AVRout (rs= −0.40, P = 0.048) showed a negative correlation with the number of CMBs.
Conclusions:
Measurement of retinal vessels using EDI-OCT correlates moderately well with MRI parameters. EDI-OCT might be a useful evaluation tool for CADASIL patients.
Keywords: Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy, Magnetic Resonance Imaging, Optical Coherence Tomography, Retinal Vessels
Introduction
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an inherited cerebral small and medium vessel disease caused by mutations in NOTCH3, which encodes Notch3 receptor expressed in vascular smooth muscle cells (VSMC).[1,2] Pathological changes include deposition of granular osmiophilic material on the surface of smooth muscle cells with subsequent fibrosis in the wall of arterioles.[3] Clinical manifestations of CADASIL include migraine with aura, subcortical ischemic events, mood disturbances, apathy, and cognitive impairment.[4] Brain magnetic resonance imaging (MRI) is used for diagnosis and follow-up in these patients. Hallmark findings include white matter hyperintensities (WMHs), small infarcts and cerebral microbleeds (CMBs).[5,6,7,8] Recently, High-resolution Magnetic Resonance was used to study the cerebral vessel in arteriosclerosis[9], moyamoaya disease[10] and CADASIL,[11] microbleed and microinfarct detection in amyloid angiopathy,[12] the changes of perivascular spaces and perforating arteries.[13] De Guio et al.,[14] have revealed loss of venous integrity in CADASIL patients using 7T-MRI.
CADASIL is a systemic arteriopathy, which is typically well-characterized in the retinal vessels. The retinal arteriolar narrowing, arteriovenous nicking, and bilateral peripapillary arteriolar sheathing are common changes seen on fundus photography examination.[15,16] Recent technological advances in optical coherence tomography (OCT) allow measurement of retinal vessel diameters and vessel wall thickness in vivo.[17] Alten et al.[18] documented a significant increase in arterial and venous outer diameters as well as arterial and venous wall thicknesses in German CADASIL patients. It needs to be confirmed if changes in retinal vessels correlate with cerebral MRI findings so that in future enhanced depth imaging (EDI)-OCT can be used as an alternative modality for a follow-up examination in these patients. In this study, we performed both EDI-OCT and high-field MRI in CADASIL patients to measure retinal vessel diameters and subfoveal choroidal thickness (SFCT). Further, the correlation between cerebral MRI findings and retinal vessel changes was investigated.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki. The institutional review board and ethics committee at the Peking University First Hospital approved this study. Written informed consent was obtained from all participants.
Patients
Twenty-seven CADASIL patients (15 male and 12 female; mean age, 44.9 ± 9.0 years, range 29–64 years), belonging to 19 families, were recruited from January 2015 to August 2016. All patients had Notch3 mutations confirmed on genetic analysis. Patients with any of the following were excluded in the study: high myopia (>−3 diopters), high astigmatism (>±3 diopters), or any concomitant ocular disease. Twenty-seven age- and sex-matched healthy controls (15 male and 12 female; mean age, 42.9 ± 8.4 years, range 31–64 years) who had no known medical conditions (e.g., diabetes, stroke, hypertension, and ischemic heart disease) or ocular diseases (glaucoma, diabetic retinopathy, age-related macular degeneration) were recruited as controls. There was no significant difference between the age of CADASIL patients and controls (t = 0.822, P = 0.117).
Complete medical history was obtained from all participants, none of the patients have hypertension or diabetes mellitus except one 61-year-old male patient has hypertension for 30 years. Disease duration was determined based on the first occurrence of neurological symptoms. The medium disease duration of the twenty-two symptomatic patients was 5.5 years (range, 0.5–12 years). Five subjects were asymptomatic, so they were excluded from the disease duration analysis. They were evaluated because of familiarity. Symptomatic stroke was the most frequent manifestation (n = 19), followed by chronic headache (n = 14), cognitive impairment (n = 7), and mood disorders (n = 6).
Brain magnetic resonance imaging examination
All patients underwent 7T-brain MRI examination (Siemens Healthcare, Germany). 7T-MRI examinations were performed as part of our ongoing “CADASIL patients’ 7T-MRI study”. Parameters of brain imaging protocol included the following: 3D T1-magnetization prepared gradient echo images (TR = 2200 ms, TE = 3.29 ms, voxel = 0.70 mm × 0.70 mm × 0.70 mm), fluid-attenuated inversion recovery images (TR = 14000 ms, TE = 94 ms, voxel = 0.43 mm × 0.57 mm × 3.00 mm), and susceptibility-weighted imaging (SWI)-gradient echo image (TR = 18 ms, TE = 12 ms, voxel = 0.30 mm × 0.30 mm × 1.20 mm). White matter lesions were scored using Fazekas scale (total score range: 0–6).[19] The location and the numbers of small infarcts were recorded.[20,21] The total number of CMBs was counted according to the Microbleed Anatomical Rating Scale.[22,23] All MRI of good quality were included for rating of brain lesions. SWI images of two patients were excluded due to artifacts introduced by head movement. The imaging analysis (presence of small infarcts and CMBs, rating of the white matter lesion) was performed by two experienced neurologists by consensus and without any knowledge of clinical information.
Enhanced depth imaging optical coherence tomography examination
All participants were examined with EDI-OCT (Heidelberg Engineering, Heidelberg, Germany). SFCT was defined as the vertical distance from the hyperreflective line of the Bruch's membrane to the hyperreflective line of the inner surface of the sclera [Figure 1a and 1b]. The measurements were performed using the Heidelberg Eye Explorer software (Heidelberg Engineering Co, Heidelberg, Germany). Both eyes of each participant were assessed. The images were taken by one technician and assessed by two ophthalmologists.
Figure 1.
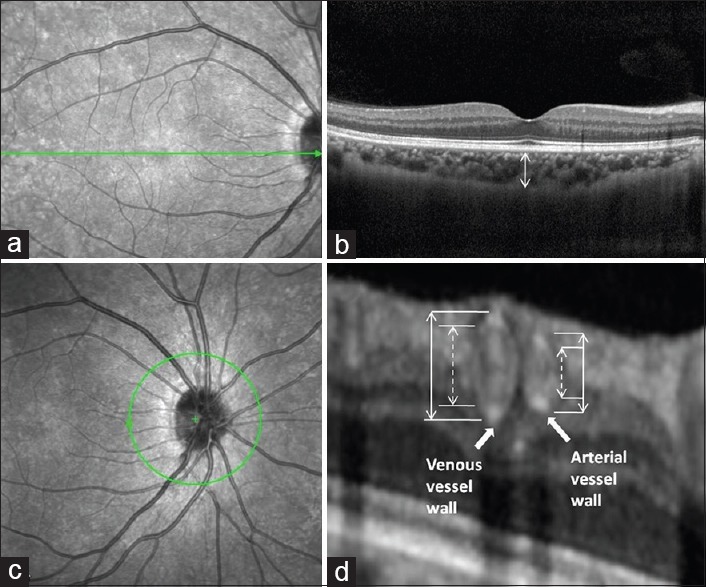
SFCT and retinal vessel measurements on EDI-OCT. Perifoveal scan of a CADASIL patient (a), green line indicates the position of the scan, which is the fovea of the right eye; (b) SFCT represents the vertical distance from the hyperreflective line of the Bruch's membrane to the hyperreflective line of the inner surface of the sclera. Peripapillary scan of a CADASIL patient (c), green circle indicates the circular scan centered on the optic disk. Cross-sectional images of all major retinal arteries and veins (d), hyper-reflectivities represent the vessel walls. Arrows indicate vessel walls. SFCT: Subfoveal choroidal thickness; EDI-OCT: Enhanced depth imaging optical coherence tomography; CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.
Retinal vessel diameter and vessel wall thickness
A circular scan (diameter = 3.5 mm) centered on the optic disc provides cross-sectional images of all major retinal arteries and veins [Figure 1c and 1d]. As reported previously, OCT light projected vertically to the vessel walls and lumen. Normal vessels had heterogeneous reflectivity, appeared oval in shape, and often had four hyperreflectivities in a linear configuration.[24] Inner and outer vessel diameters of the four largest retinal arteries and veins, vessel wall thickness ([outer vessel diameter plus minor inner vessel diameter]/2), arterial inner diameter/venous inner diameter (AVRin), and arterial outer diameter/venous outer diameter (AVRout) were measured by two raters who were blinded to the clinical information [Figure 1c and 1d]. Three veins were excluded in CADASIL group due to questionable border discrimination of vessel walls.
Statistical analysis
Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). SFCT and vessel diameter values are presented as mean ± standard deviation (SD). Paired sample t-test was used to compare the SFCT and retinal vessel measurement data between patients and controls. Both eye values were used for statistical comparisons between the groups. The eye with the most obvious changes was chosen for each CADASIL patient to draw a correlation with the corresponding MRI lesions. The bivariate relationships between MRI lesions and retinal vessel measurements were analyzed using Spearman's correlation coefficient. Statistical significance was defined as P < 0.05.
Results
Subfoveal choroidal thickness, retinal vessel diameter, and vessel wall thickness measurements on enhanced depth imaging optical coherence tomography
Mean SFCT in CADASIL patients was significantly lower than that in controls. Mean arterial outer diameter and mean venous outer diameter in CADASIL patients was significantly larger than that in controls. The mean arterial inner diameter in CADASIL patients was significantly smaller than that in controls, while the mean venous inner diameter in CADASIL patients was significantly larger than that in controls. The mean vessel wall thickness of arteries and veins in CADASIL patients were significantly greater than those in controls [Table 1 and Figure 2]. The AVRout and AVRin in CADASIL patients were significantly smaller than those in controls [Table 1].
Table 1.
Comparison of retinal vessel parameters and SFCT between CADASIL patients and healthy controls
| Vessel parameters | CADASIL (n = 27) | Control (n = 27) | t | P |
|---|---|---|---|---|
| SFCT (µm) | 268.37 ± 46.50* | 300.53 ± 39.31 | −3.401 | <0.001 |
| Arteries (µm) | ||||
| Inner diameter | 93.46 ± 9.70* | 96.94 ± 8.28 | −2.068 | 0.048 |
| Outer diameter | 131.74 ± 10.87* | 127.36 ± 8.67 | 2.224 | 0.023 |
| Vessel wall | 19.13 ± 1.85* | 15.21 ± 1.47 | 12.367 | <0.001 |
| Veins (µm) | ||||
| Inner diameter | 128.99 ± 13.62† | 121.97 ± 11.29 | 2.737 | 0.004 |
| Outer diameter | 164.82 ± 14.77† | 149.60 ± 11.96 | 5.414 | <0.001 |
| Vessel wall | 17.91 ± 2.76† | 13.81 ± 1.47 | 9.656 | <0.001 |
| AVR | ||||
| AVRin | 0.72 ± 0.08† | 0.79 ± 0.07 | −5.718 | <0.001 |
| AVRout | 0.80 ± 0.06† | 0.85 ± 0.06 | −4.904 | <0.001 |
Data are presented as mean ± SD. *Means the SFCT and arterial parameters in CADASIL patients (n = 54) versus control (n = 54); †Means the venous parameters and AVR data in CADASIL patients (n = 51) versus control (n = 51); Three veins were excluded in CADASIL group due to questionable border discrimination of vessel walls; Paired t-test was used to analyze the date of normal distribution. SFCT: Subfoveal choroidal thickness; CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; AVRin: Arterial venous ratio of inner diameter; AVRout: Arterial venous ratio of outer diameter; SD: Standard deviation.
Figure 2.
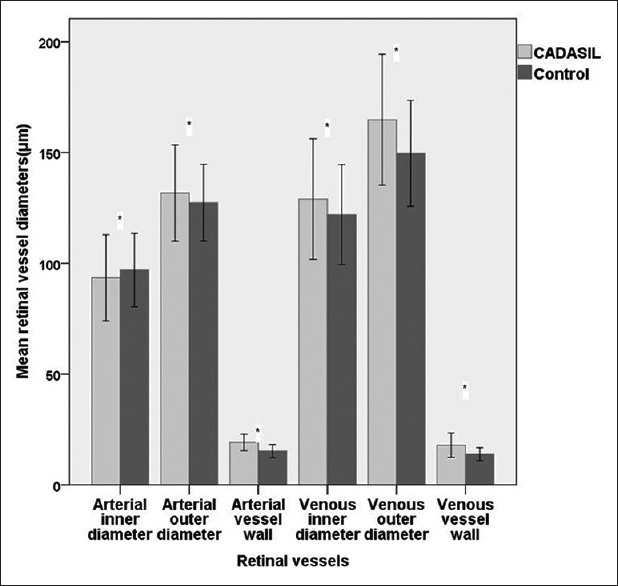
Comparison of retinal vessel parameters between CADASIL patients and controls. *Means the data in CADASIL group versus that in control group, P < 0.05. CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. (n=27 each group).
Magnetic resonance imaging findings
In addition of one asymptomatic case in early stage of the disease (age 27 years), the white matter lesions were observed in 26/27 (96.0%) patients [Figure 3a] including involvement of the temporal lobe in 25/27 (92.0%) patients and involvement of the external capsule in 20/27 (74.0%) patients. The Fazekas score was 4.14 ± 1.36. Infarcts with diameter ranging from 1.4 mm to 15.0 mm were observed in 23/27 (85.0%) patients; these were distributed in the subcortical white matter (42.3%), basal ganglia (30.2%), thalamus (16.4%), brain stein (10.0%), cerebellum (1.0%), and the cortex [Figure 3b and 3c]. The median number of small infarcts was 15. Two SWI scans were excluded because of insufficient quality, leaving 25 participants for evaluation. CMBs were observed in 13/25 patients (52.0%), with a median number of 3. CMBs were most commonly located in thalamus (41.8%), cortex (24.7%) (6.5% in frontal, 5.9% in temporal, 8.8% in parietal, 1.2% in occipital, and 1.2% in insular lobe), and basal ganglia (17.6%) [Figure 3d].
Figure 3.
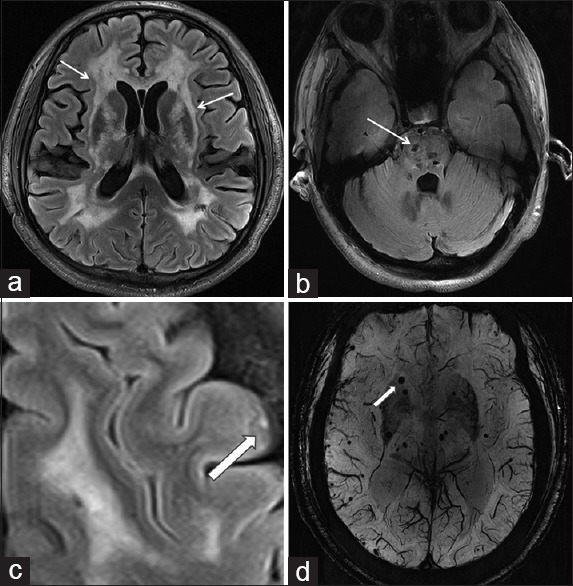
MRI of CADASIL patients. FLAIR image of a CADASIL patient (a), arrows indicate external capsule white matter lesions and periventricular white matter lesions. Lacunar infarcts in the pons (b), arrow indicates hypointense in the pons. Cortical microinfarct on FLAIR image (c), arrow indicates hyperintense microinfarcts on the cortex. Cerebral microbleeds in basal ganglia and cerebral cortex (d), arrow showing small, round, hypointense microbleeds on SWI-gradient echo image. MRI: Magnetic resonance imaging; CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; Flair: Fluid-attenuated inversion recovery; SWI: Susceptibility-weighted imaging.
Phenotype-genotype correlation
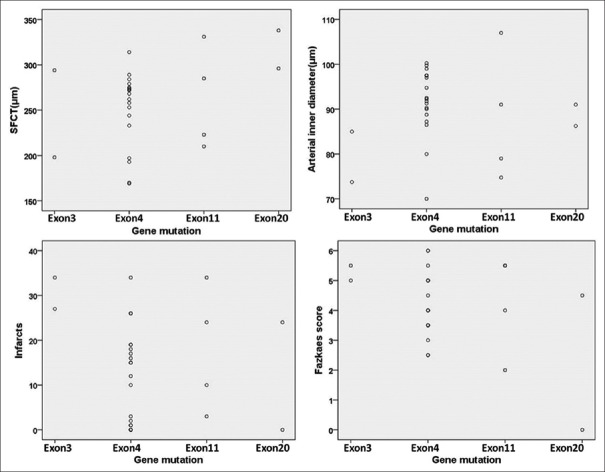
The NOTCH3 gene mutation of these 27 patients showed 18 mutations, with hotspots in exon 4 (n = 19, 70.4%), followed by exon 11 (n = 4, 14.8%), exon 3 (n = 2, 7.4%), and exon 20 (n = 2, 7.4%). The considerable variability in retinal vessel measurements and MRI lesions in patients carrying a mutation in the same exon suggested no obvious phenotype-genotype correlation [Figure 4].
Figure 4.
CADASIL patients with mutation of the same exon had a greater variability in retinal vessel measurements and MRI lesions. CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; MRI: Magnetic resonance imaging; SFCT: Subfoveal choroidal thickness (n=27).
Correlation between brain magnetic resonance imaging lesions and retinal vessel measurements
The arterial inner diameters, AVRin and AVRout showed a significant negative correlation with the number of small infarcts. The venous inner diameter showed a significant positive correlation with the number of small infarcts. The venous inner and outer diameters showed a significant positive correlation with CMB numbers. AVRin and AVRout showed a significant negative correlation with CMB numbers. Neither the diameter of the retinal vessels nor the AVR correlated with white matter Fazekas score. There was no correlation between SFCT and the number of small infarcts, CMB numbers and Fazekas score in CADASIL patients [Table 2].
Table 2.
Correlation between retinal vessel measurements and MRI findings in CADASIL patients (n = 27)
| Vessel parameters | Infarcts | Fazkas score | CMBs | |||
|---|---|---|---|---|---|---|
| rs | P | rs | P | rs | P | |
| SFCT | −0.05 | 0.812 | 0.26 | 0.195 | 0.26 | 0.203 |
| Arterial inner diameter | −0.39* | 0.044 | −0.22 | 0.267 | 0.02 | 0.926 |
| Arterial outer diameter | 0.21 | 0.303 | 0.09 | 0.661 | 0.13 | 0.548 |
| Arterial vessel wall | 0.23 | 0.257 | 0.25 | 0.201 | 0.07 | 0.751 |
| Venous inner diameter | 0.46* | 0.016 | 0.02 | 0.897 | 0.59* | 0.002 |
| Venous outer diameter | 0.32 | 0.104 | −0.12 | 0.561 | 0.47* | 0.017 |
| Venous vessel wall | 0.09 | 0.665 | −0.17 | 0.386 | 0.11 | 0.595 |
| AVRin | −0.65* | <0.001 | −0.30 | 0.125 | −0.52* | 0.007 |
| AVRout | −0.56* | 0.002 | 0.08 | 0.691 | −0.40* | 0.048 |
The bivariate relationships between MRI lesions and retinal vessel measurements were analyzed using Spearman’s correlation. *Means the data of retinal vessel parameters correlate with brain lesions, P<0.05. MRI: Magnetic resonance imaging; CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CMBs: Cerebral microbleeds; SFCT: Subfoveal choroidal thickness; AVRin: Arterial venous ratio of inner diameter; AVRout: Arterial venous ratio of outer diameter.
Discussion
We used SFCT to evaluate the choroid because it is highly reproducible and is widely used in other small-vessel diseases.[25,26,27] The present study revealed reduced SFCT in CADASIL patients. Young adults with systemic arterial hypertension were shown to have significantly lower SFCT as compared to that in healthy controls.[25] Thinner SFCT is also seen in diabetic patients without diabetic retinopathy,[26,27] and in patients with migraine.[28] In addition, a decrease in mean SFCT was also described in ocular ischemic syndrome.[29] Although no other information was available on SFCT measurement in CADASIL patients, these results are consistent with those reported by Robinson et al.,[30] they found irregular choroidal filling on fundus fluorescein angiography. We hypothesize that reduced SFCT might be a common change in cerebral microvascular disease, the underlying pathophysiological mechanism of which is not clear and might be attributable to reduced vascular diameter or hypoperfusion of the vessels.
We further confirmed that the outer diameters of arteries and veins were significantly larger than those in healthy controls. Since the vessel wall was significantly thicker in veins and arteries, the inner diameters of arteries in CADASIL patients was significantly lower and which narrowed the arterial lumen. This finding is also consistent with that of a pathological study that CADASIL is characterized by thickening of the arterial wall leading to luminal stenosis.[31] Similar findings for outer diameters and vessel wall in arteries and veins were reported in CADASIL patients by Alten et al.[18] However, Alten et al.[18] found no significant difference of arterial inner diameter between CADASIL patients and controls. Muraoka et al.[24] reported an increase in both mean arterial and venous wall thickness in retinal vessels of patients with hypertension. However, the arterial inner diameters, venous inner and outer diameters did not show a significant difference from that in healthy controls. Compared to patients with hypertension, CADASIL patients also showed enlarged inner and outer diameters of veins. We suggest that small veins in patients with CADASIL might also be altered. The mechanism and significance of venous involvement need further study. Narrowing of retinal arterial lumina and larger venous lumina were associated with the severity of infarcts. This is in accordance with former studies performed using fundus photography, which showed an association of arterial narrowing with a greater risk of infarcts and leukoaraiosis.[32,33] We also observed a relationship between venular dilation (both inner and outer diameter) and the severity of small infarcts and CMBs. A possible explanation might be that retinal venular dilation reflects cerebral venular function abnormalities. CMBs can be caused by high venous pressure, which is usually associated with venular dilation and disruption of the blood-brain barrier. In the Rotterdam Scan Study, larger retinal venular diameters were associated with progression of white matter lesions, but not with the severity of leukoaraiosis.[34] Leukoaraiosis is no specific change which can be found in many diseases, such as mitochondrial disease.[35] The temporal pole involvement appeared also in myotonia dystrophy type 1.[36] The severity of leukoaraiosis was found to be related to the loss of cerebral venous integrity in CADASIL patients using 7T-MRI.[14] Therefore, we should pay more attention to the venous functional and morphological changes in CADASIL.
In this study, we used the conventional MRI evaluation index to evaluate brain lesions in CADASIL, due to which we cannot show the superiority of high field strength MRI.[37] Small sample size was another limitation of the study. However, the results might reflect the changes in CADASIL patients.
In conclusion, we used EDI-OCT examination to confirm the decrease in SFCT and retinal artery lumina in Chinese CADASIL patients. Retinal artery outer diameter, venous inner and outer diameter and vessel wall thickness of both arteries and veins was increased in CADASIL patients. Narrowing of the retinal arterial lumen is related to infarcts and dilation of vein related to CMBs. The measurement of retinal vessels using EDI-OCT is a useful evaluation tool for follow-up of CADASIL patients.
Financial support and sponsorship
This study was supported by grants from the National Key Research and Development Program of China (No. 2016YFC1300600), National Natural Science Foundation of China (No. 81471185), and National Science and Technology Major Project (No. 2011ZX09307-001-07).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank our technicians Hai-Long Wu, and Ya-Di Zhang for their help with data collection.
Footnotes
Edited by: Peng Lyu
References
- 1.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–10. doi: 10.1038/383707a0. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Zuo Y, Sun W, Zhang W, Lv H, Huang Y, et al. The genetic spectrum and the evaluation of CADASIL screening scale in Chinese patients with NOTCH3 mutations. J Neurol Sci. 2015;354:63–9. doi: 10.1016/j.jns.2015.04.047. doi: 10.1016/j.jns.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 3.Tikka S, Baumann M, Siitonen M, Pasanen P, Pöyhönen M, Myllykangas L, et al. CADASIL and CARASIL. Brain Pathol. 2014;24:525–44. doi: 10.1111/bpa.12181. doi: 10.1111/bpa.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao YC, Hsiao CT, Fuh JL, Chern CM, Lee WJ, Guo YC, et al. Characterization of CADASIL among the Han Chinese in Taiwan: Distinct genotypic and phenotypic profiles. PLoS One. 2015;10:e0136501. doi: 10.1371/journal.pone.0136501. doi: 10.1371/journal.pone.0136501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stojanov D, Vojinovic S, Aracki-Trenkic A, Tasic A, Benedeto-Stojanov D, Ljubisavljevic S, et al. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) Bosn J Basic Med Sci. 2015;15:1–8. doi: 10.17305/bjbms.2015.247. doi: 10.17305/bjbms.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yagi T, Konoeda F, Mizuta I, Mizuno T, Suzuki N. Increasing microbleeds in CADASIL. Eur Neurol. 2013;69:352–3. doi: 10.1159/000348720. doi: 10.1159/000348720. [DOI] [PubMed] [Google Scholar]
- 7.O’Sullivan M, Jarosz JM, Martin RJ, Deasy N, Powell JF, Markus HS. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001;56:628–34. doi: 10.1212/wnl.56.5.628. doi: 10.1212/WNL.56.5.628. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto Y, Ihara M, Tham C, Low RW, Slade JY, Moss T, et al. Neuropathological correlates of temporal pole white matter hyperintensities in CADASIL. Stroke. 2009;40:2004–11. doi: 10.1161/STROKEAHA.108.528299. doi: 10.1161/STROKEAHA.108.528299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Le WJ, Tao XF, Li MH, Li YH, Qu N. Advantage in Bright-blood and Black-blood Magnetic Resonance Imaging with High-resolution for Analysis of Carotid Atherosclerotic Plaques. Chin Med J. 2015;128:2478–84. doi: 10.4103/0366-6999.164933. doi: 10.4103/0366-6999.164933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu LB, Zhang Q, Shi ZY, Wang MQ, Zhang D. High-resolution Magnetic Resonance Imaging of Moyamoya Disease. Chin Med J. 2015;128:3231–7. doi: 10.4103/0366-6999.170257. doi: 10.4103/0366-6999.170257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liem MK, Lesnik Oberstein SA, Versluis MJ, Maat-Schieman ML, Haan J, Webb AG, et al. 7 T MRI reveals diffuse iron deposition in putamen and caudate nucleus in CADASIL. J Neurol Neurosurg Psychiatry. 2012;83:1180–5. doi: 10.1136/jnnp-2012-302545. doi: 10.1136/jnnp-2012-302545. [DOI] [PubMed] [Google Scholar]
- 12.van Veluw SJ, Charidimou A, Aj VDK, Lauer A, Reijmer YD, Costantino I, et al. Microbleed and microinfarct detection in amyloid angiopathy: A high-resolution mri-histopathology study. Brain. 2016;139:3151. doi: 10.1093/brain/aww229. doi: 10.1093/brain/aww229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouvy WH, Biessels GJ, Kuijf HJ, Kappelle LJ, Luijten PR, Zwanenburg JJ. Visualization of perivascular spaces and perforating arteries with 7 t magnetic resonance imaging. Investigative Radiology. 2014;49:307. doi: 10.1097/RLI.0000000000000027. doi:10.1097/RLI.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 14.De Guio F, Vignaud A, Ropele S, Duering M, Duchesnay E, Chabriat H, et al. Loss of venous integrity in cerebral small vessel disease: A 7-T MRI study in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) Stroke. 2014;45:2124–6. doi: 10.1161/STROKEAHA.114.005726. doi: 10.1161/STROKEAHA.114.005726. [DOI] [PubMed] [Google Scholar]
- 15.Haritoglou C, Rudolph G, Hoops JP, Opherk C, Kampik A, Dichgans M. Retinal vascular abnormalities in CADASIL. Neurology. 2004;62:1202–5. doi: 10.1212/01.wnl.0000118296.16326.e1. doi: 10.1212/01.WNL.0000118296.16326.E1. [DOI] [PubMed] [Google Scholar]
- 16.Roine S, Harju M, Kivelä TT, Pöyhönen M, Nikoskelainen E, Tuisku S, et al. Ophthalmologic findings in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: A cross-sectional study. Ophthalmology. 2006;113:1411–7. doi: 10.1016/j.ophtha.2006.03.030. doi: 10.1016/j.ophtha.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Goldenberg D, Shahar J, Loewenstein A, Goldstein M. Diameters of retinal blood vessels in a healthy cohort as measured by spectral domain optical coherence tomography. Retina. 2013;33:1888–94. doi: 10.1097/IAE.0b013e31829477f2. doi: 10.1097/IAE.0b013e31829477f2. [DOI] [PubMed] [Google Scholar]
- 18.Alten F, Motte J, Ewering C, Osada N, Clemens CR, Kadas EM, et al. Multimodal retinal vessel analysis in CADASIL patients. PLoS One. 2014;9:e112311. doi: 10.1371/journal.pone.0112311. doi: 10.1371/journal.pone.0112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–6. doi: 10.2214/ajr.149.2.351. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 20.Norrving B. Evolving concept of small vessel disease through advanced brain imaging. J Stroke. 2015;17:94–100. doi: 10.5853/jos.2015.17.2.94. doi: 10.5853/jos.2015.17.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardlaw J M. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration[J] Lancet Neurology. 2013;12:822. doi: 10.1016/S1474-4422(13)70124-8. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009;8:165–74. doi: 10.1016/S1474-4422(09)70013-4. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, et al. The Microbleed Anatomical Rating Scale (MARS): Reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–66. doi: 10.1212/WNL.0b013e3181c34a7d. doi: 10.1212/WNL.0b013e3181c34a7d. [DOI] [PubMed] [Google Scholar]
- 24.Muraoka Y, Tsujikawa A, Kumagai K, Akiba M, Ogino K, Murakami T, et al. Age- and hypertension-dependent changes in retinal vessel diameter and wall thickness: An optical coherence tomography study. Am J Ophthalmol. 2013;156:706–14. doi: 10.1016/j.ajo.2013.05.021. doi: 10.1016/j.ajo.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Akay F, Gundogan FC, Yolcu U, Toyran S, Uzun S. Choroidal thickness in systemic arterial hypertension. Eur J Ophthalmol. 2016;26:152–7. doi: 10.5301/ejo.5000675. doi: 10.5301/ejo.5000675. [DOI] [PubMed] [Google Scholar]
- 26.Querques G, Lattanzio R, Querques L, Del Turco C, Forte R, Pierro L, et al. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53:6017–24. doi: 10.1167/iovs.12-9692. doi: 10.1167/iovs.12-9692. [DOI] [PubMed] [Google Scholar]
- 27.Kim JT, Lee DH, Joe SG, Kim JG, Yoon YH. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2013;54:3378–84. doi: 10.1167/iovs.12-11503. doi: 10.1167/iovs.12-11503. [DOI] [PubMed] [Google Scholar]
- 28.Zengin MO, Elmas Z, Cinar E, Kucukerdonmez C. Choroidal thickness changes in patients with migraine. Acta Neurol Belg. 2015;115:33–7. doi: 10.1007/s13760-014-0301-3. doi: 10.1007/s13760-014-0301-3. [DOI] [PubMed] [Google Scholar]
- 29.Kim DY, Joe SG, Lee JY, Kim JG, Yang SJ. Choroidal thickness in eyes with unilateral ocular ischemic syndrome. J Ophthalmol 2015. 2015 doi: 10.1155/2015/620372. 620372. doi: 10.1155/2015/620372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson W, Galetta SL, McCluskey L, Forman MS, Balcer LJ. Retinal findings in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (cadasil) Surv Ophthalmol. 2001;45:445–8. doi: 10.1016/s0039-6257(00)00206-x. doi: 10.1016/S0039-6257(00)00206-X. [DOI] [PubMed] [Google Scholar]
- 31.Miao Q, Paloneva T, Tuominen S, Pöyhönen M, Tuisku S, Viitanen M, et al. Fibrosis and stenosis of the long penetrating cerebral arteries: The cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathol. 2004;14:358–64. doi: 10.1111/j.1750-3639.2004.tb00078.x. doi: 10.1111/j.1750-3639.2004.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung N, Mosley T, Islam A, Kawasaki R, Sharrett AR, Klein R, et al. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: A prospective study. Brain. 2010;133(Pt 7):1987–93. doi: 10.1093/brain/awq127. doi: 10.1093/brain/awq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Wu Y, Xie S, Luan XH, Yuan Y. Retinal arterial abnormalities correlate with brain white matter lesions in cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy. Clin Exp Ophthalmol. 2008;36:532–6. doi: 10.1111/j.1442-9071.2008.01825.x. doi: 10.1111/j.1442-9071.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 34.Ikram MK, De Jong FJ, Van Dijk EJ, Prins ND, Hofman A, Breteler MM, et al. Retinal vessel diameters and cerebral small vessel disease: The Rotterdam Scan Study. Brain. 2006;129(Pt 1):182–8. doi: 10.1093/brain/awh688. doi: 10.1093/brain/awh688. [DOI] [PubMed] [Google Scholar]
- 35.Yu M, Zhang Z, Wang QQ, Liu J, Zuo YH, Yu L, et al. Clinical and Brain Magnetic Resonance Imaging Features in a Cohort of Chinese Patients with Kearns-Sayre Syndrome. Chin Med J. 2016;129:1419–24. doi: 10.4103/0366-6999.183417. doi: 10.4103/0366-6999.183417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Liu HM, Liu ZJ, Zhang LW, Gu WH, Wang RB. Myotonic dystrophy type 1 associated with white matter hyperintense lesions: clinic, imaging, and genetic analysis. Chin Med J. 2015;128:1412–4. doi: 10.4103/0366-6999.156812. doi: 10.4103/0366-6999.156812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu XJ, Wang W, Liu ZJ. High-resolution magnetic resonance vessel wall imaging for intracranial arterial stenosis. Chin Med J. 2016;129:1363–70. doi: 10.4103/0366-6999.182826. doi: 10.4103/0366-6999.182826. [DOI] [PMC free article] [PubMed] [Google Scholar]