Abstract
Portal vein thrombosis (PVT) is frequent in patients with liver cirrhosis and possible severe complications such as mesenteric ischemia are rare, but can be life-threatening. However, different aspects of clinical relevance, diagnosis and management of PVT are still areas of uncertainty and investigation in international guidelines. In this article, we elaborate on PVT classification, geographical differences in clinical presentation and standards of diagnosis, and briefly on the current pathophysiological understanding and risk factors. This review considers and highlights the pitfalls of the various treatment approaches and prophylactic treatments. Finally, we review the controversial issue of clinical impact of PVT on prognosis, especially considering liver transplantation and future perspectives.
Keywords: portal vein thrombosis, liver cirrhosis, thrombophilia tests, low-molecular-weight heparin, transjugular intrahepatic portosystemic shunt, liver transplantation
Introduction
Portal vein thrombosis (PVT) in the general population is a rare event, but it occurs relatively frequently in patients with liver cirrhosis and its prevalence increases with the severity of the disease. PVT can develop in the intra- or extrahepatic segments of the portal vein and extend to the superior mesenteric vein and/or the splenic vein. Moreover, PVT can progress from a partial obstruction due to the presence of a thrombus in the lumen of the vein to a complete blockade of portal venous blood flow. In cirrhotic patients, the prevalence of PVT ranges between 0.6% and 26% [1].
However, prevalence can vary depending on age, underlying hepatic disease, velocity of portal venous blood flow and the pro- versus anticoagulant status of the patient. In patients with cirrhosis of Child-Pugh A and B, incidence of newly diagnosed PVT after 1 and 5 years has been reported to be 4.6% and 10.7%, respectively [2]. The relative risk of developing PVT in the presence of cirrhosis is increased more than seven-fold above the risk observed in the general population, which is estimated to be < 1.0% [3]. While patients with compensated cirrhosis are rarely affected, PVT is frequently detected in advanced stages, increasing up to 25% in liver-transplantation candidates and 35% in cirrhotic patients with hepatocellular carcinoma (HCC) [4]. However, in large studies including patients evaluated for liver transplantation, 6.3% were diagnosed with PVT, in particular when cirrhosis was related to nonalcoholic steatohepatitis [5]. Moreover, in patients with advanced cirrhosis and those undergoing liver transplantation, a prevalence of between 5% and 16% has been reported [6].
The signs and symptoms of PVT are very heterogeneous and range from incidental diagnosis during diagnostic procedures for unrelated reasons to severe complications due to intestinal infarction or to the development of portal hypertension, such as variceal bleeding that can occur from esophageal and/or gastric fundic varices when splenic vein thrombosis is present. Therefore, prognosis and treatment of PVT depend on the localization, the degree of extension and the rapidity of development, as well as risk factors for thrombosis and the stage of chronic advanced liver disease.
The causal relationships and the clinical presentation are often complex. As PVT may cause short-term as well as long-term complications, correct management by adopting adequate diagnostic and therapeutic measures is paramount. In this review article, we discuss the classification, the symptoms and signs, and the pathogenesis of PVT in cirrhosis, its clinical relevance, diagnostic procedures and the main lines of management.
Definitions and classification
PVT refers to partial or complete occlusion by a blood clotting of the portal vein trunk that can also include its right and left intrahepatic branches. Extension to either superior mesenteric or splenic veins or secondary intrahepatic portal branches is possible.
However, the definitions show geographic differences. In European guidelines, recent PVT has been defined as a recent formation of a thrombus within the portal vein and/or right or left branches [7]. In the absence of recanalization, the portal venous lumen is obliterated, while porto-portal collaterals develop, resulting in the cavernomatous transformation of the portal vein, which might represent chronic PVT. The American Association for the Study of Liver Diseases (AASLD), however, defines acute PVT as the sudden formation of a thrombus within the portal vein lumen, and chronic PVT when the obstructed portion is replaced by a network of hepato-petal collaterals bypassing the thrombosed portion of the portal vein [8]. Although these definitions are easily interpreted and practice-oriented, they are based purely on anatomic findings and are limited by the fact that they only consider occlusion as defining criteria, omitting any clinically significant consequences.
In patients with liver cirrhosis, PVT may be more accurately defined as a clinical syndrome that presents either as an incidental finding on abdominal imaging (performed for reasons unrelated to PVT) or with highly variable signs and symptoms resulting from portal vein obstruction and/or portal hypertension [9].
PVT in patients with cirrhosis has been shown to have little influence on the course of liver disease, except for patients with PVT at liver transplantation in whom 90-day mortality and graft failure have been reported to be higher than in those without PVT [10,11]. Moreover, non-progression or resolution has been reported in up to 75% of patients. In general, PVT might be the cause as well as the consequence of decompensation of cirrhosis or advanced liver disease.
To predict the presence of PVT in patients with cirrhosis, some factors have been suggested, including Child B and C stage, prior history of resolved PVT, associated pro-thrombotic risk factors (major characteristics) and large portosystemic collaterals, HCC, history of systemic venous thrombosis, recent abdominal surgical, endoscopic or invasive interventions and portal flow velocity < 15 cm/sec [9].
To date, several classification systems for PVT have been proposed but, despite their easy applicability, they failed to assess PVT with precision, including site, degree, presentation and functional relevance of the thrombosis [12–15]. Therefore, a comprehensive new classification has been proposed that includes not only an anatomical description, but also additional information that will support the management decision process [9]. The features of this new classification are presented in Table 1.
Table 1.
New classification of portal vein thrombosis
|
|
|
|
| Type and presence of underlying liver disease |
| Cirrhotic, non-cirrhotic liver disease, post-liver transplant, hepatocellular carcinoma, local malignancies and associated conditions |
The application of this new classification will enable a more accurate assessment of patients diagnosed with this heterogeneous condition, while its validation in prospective studies will allow refinements that will improve its relevance in the management of these patients.
Clinical presentation
In cirrhotic patients, PVT often is clinically unapparent and diagnosed incidentally by ultrasound during routine follow-up screening for HCC. In other cases, however, patients may present with life-threatening complications. It is often difficult to interpret specific symptoms, since the occurrence of PVT is not necessarily causal for the deterioration, but might be superimposed on it. Therefore, clinical deterioration may be the consequence of either a recent development of PVT or an episode of decompensation due to progression of cirrhosis.
As mentioned above, PVT can be classified as recent or chronic. Yet, this differentiation is based on clinical findings and imaging rather than on temporal criteria. Although some authors consider PVT as acute if symptoms developed less than 60 days prior to hospitalization [16], this time duration is arbitrary and particularly difficult to verify.
In a study including patients with cirrhosis and cancer, the patients presented with abdominal symptoms (including abdominal pain, loss of appetite, nausea, vomiting and diarrhea) (63%), splenomegaly (63%), gastrointestinal (GI) hemorrhage (including hematemesis, rectal bleeding or melena) (53%), fever (37%), ascites (32%) and weight loss (16%) [17]. Of these, abdominal symptoms and fever were more frequent in acute PVT, while gastroesophageal varices and hemorrhages occurred more frequently in patients with chronic PVT.
In another study with 701 cirrhotic patients, 11.2% of patients developed PVT and 57% of them presented with symptoms including GI bleeding (due to varices or portal hypertensive gastropathy) or abdominal pain [18]. Acute abdominal pain was frequently caused by intestinal ischemia or infarction due to thrombotic involvement of the mesenteric veins. Moreover, the majority of PVT occurred in patients with advanced cirrhosis, i.e. Child-Pugh stage B or C.
The clinical presentation of acute PVT varies in relation to the extension of the thrombus. Involvement of the mesenteric veins and its branches may lead to intestinal ischemia or infarction. As intestinal infarction has a mortality rate of up to 60%, this may become fatal without rapid, correct diagnosis and adequate treatment. An accurate diagnosis, however, is often difficult, because the clinical manifestations are entirely non-specific. The diagnosis of intestinal infarction must be considered when symptoms include persistent severe abdominal pain or ileus, intestinal bleeding, ascites or organ failure. In the presence of lactic acidosis and increased inflammatory laboratory findings (elevated C-reactive protein and leukocytosis), a surgical evaluation is mandatory, as bowel resection could be indicated [7].
The clinical presentation of chronic PVT is often characterized by the consequences of portal hypertension, including gastroesophageal varices, GI bleeding, splenomegaly and hypersplenism. Cirrhotic patients with PVT seem to be exposed to a higher risk of variceal bleeding than cirrhotic patients without PVT [1].
The clinical impact of PVT on the natural course of the disease has been the subject of many studies. Luca et al. studied the association between PVT progression or regression and the clinical outcome [19]. The luminal obstruction may be partial or complete, depending on the severity of the thrombosis. In most patients, PVT was partial at diagnosis, but thrombotic aspects changed over time. Compared to patients with spontaneous regression of PVT, thrombotic progression did not correlate with an increase in mortality, episodes of hepatic decompensation and need for liver transplantation, at least in cirrhotic patients with non-malignant PVT. Nery et al. found that, in patients with cirrhosis, the development of PVT did not follow a recent progression of liver disease and that there was no evidence that the development of PVT was responsible for further progression of liver disease [2]. A recent study confirmed that PVT does not seem to worsen the prognosis of cirrhosis [20].
Diagnosis
The diagnosis of PVT is based on imaging techniques, including ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI). In addition to direct demonstration of the thrombus, further radiological aspects may indicate recent or chronic onset, pathogenic mechanisms or possible complications.
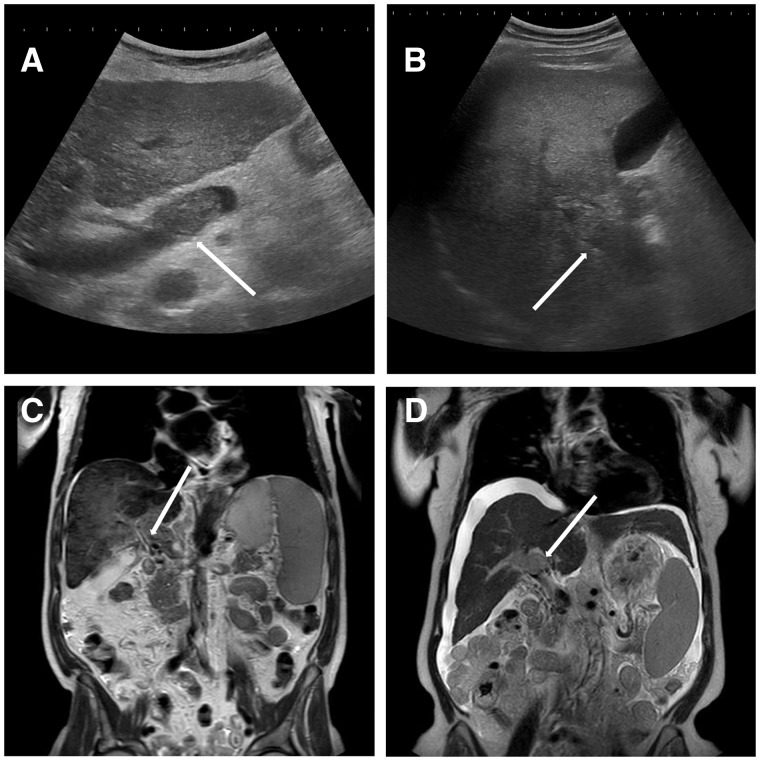
Doppler ultrasound is the first-choice diagnostic method, as it is widely available, rapid and cheap. The thrombus can be made visible as solid isoechoic or hyperechoic material within the portal vein (Figure 1). In the early stages, however, the thrombus may appear hypoechoic. With this method, it is possible to accurately evaluate whether the luminal obstruction is partial or complete. Detection of a portal cavernoma (as multiple serpiginous para-portal vessels) is highly suggestive for chronic PVT. Portal hemodynamics, including the absence or reduction in portal vein blood flow, can be assessed by color Doppler ultrasound, pulsed wave ultrasound or, alternatively, by contrast-enhanced ultrasound (CEUS). The latter can provide more reliable results in detecting the absence of flow and in patients with an extreme reduction in portal vein flow velocity [21].
Figure 1.
Imaging findings of portal vein thrombosis. (A) An ultrasound study of the liver of a female patient with liver cirrhosis Child-Pugh C, which revealed echogenic thrombosis (arrow) in the lumen of portal vein trunk. Upon further investigation, a hepatocellular carcinoma (HCC) was diagnosed. (B) Another ultrasound study of the liver of a male patient with liver cirrhosis Child-Pugh B also revealing echogenic thrombosis (arrow) in the lumen of the portal vein trunk and intrahepatic right portal branch. Further investigation showed a mutation in JAK2-V617F-gene. (C) A CT scan of a male patient with alcohol-related liver cirrhosis and portal cavernoma (arrow). (D) A CT scan of a female patient with chronic hepatitis C infection and development of portal vein thrombosis due to HCC (arrow).
Contrast-enhanced CT or MRI are frequently used, particularly in their portal venous phase, to complete the diagnosis and extend imaging to the venous branches that may not be easily accessed by ultrasound, and to detect possible complications, including intestinal infarction. Due to better representability of the mesenteric veins, these techniques have been proven to be superior to Doppler ultrasound for the evaluation of thrombotic extension within the portal venous system [8].
In patients with HCC, tumor invasion of the portal vein should always be ruled out. Some characteristics, including increased diameter of the portal vein (≥ 23 millimeters), intrathrombotic arterial neovascularization and enhancement of endoluminal material during the arterial phase of contrast injection (detected by CT scan or CEUS) seem to allow the differentiation between benign and malignant PVT [22]. In addition to CT imaging, CEUS appears to be a highly sensitive and specific method in the diagnosis of neoplastic PVT in cirrhotic patients [23].
Once PVT has been diagnosed, screening for pro-thrombotic conditions should be considered. Abnormal results in the classic coagulation tests of prothrombin time and activated partial thromboplastin time may be misleading in the evaluation of the coagulation system, as they are inadequate for the assessment of an imbalance of pro- and anticoagulant factors in cirrhotic patients. This is due to a much higher sensitivity to the pro-coagulant factors than to the anticoagulant factors (protein C, protein S and anti-thrombin), thus predicting hypocoagulability irrespective of possible pro-thrombotic conditions. Thrombin-generation assays (TGA) and thromboelastometry appear to be more suitable for the evaluation of the actual hemostasis in cirrhosis, as their results reflect pro- as well as anticoagulant factors. However, their use in the clinical setting remains controversial and requires further evaluation [24].
Data on the benefits of screening for pro-thrombotic conditions in patients with well-compensated cirrhosis and PVT remain limited [8]. According to the European Association for the Study of the Liver (EASL) clinical practice guidelines, screening for underlying thrombotic conditions in cirrhotic patients with PVT should be considered (see below). All patients with chronic PVT should be screened endoscopically for gastroesophageal varices according to the current Baveno guidelines for patients with cirrhosis [15].
Risk factors
The pathogenesis of PVT is multifactorial. Various pro-thrombotic conditions, including local and systemic factors, associated with PVT have been identified. Hypercoagulability, reduction in portal blood flow and vascular endothelial lesions are the main risk factors for development of PVT in cirrhosis, as they are for venous thromboembolism in general (known as the Virchow’s triad).
Due to thrombocytopenia and prolonged prothrombin time, cirrhosis has long been considered to be associated with hemorrhagic coagulopathies. However, recent studies indicated a tendency towards a more balanced coagulation in cirrhosis, whereby pro-coagulant and anticoagulant factors maintain a fragile equilibrium [25]. Hypercoagulability may be explained by an increased ratio between elevated levels of factor VIII (pro-coagulative effects) and decreased levels of protein C (anti-coagulative effects) [24]. These imbalanced plasma levels of pro-coagulant and anticoagulant factors are frequently detected in cirrhotic patients and may lead to the development of PVT. The recommended thrombophilia tests are:
protein C activity;
protein S activity;
antithrombin activity;
factor-V-Leiden gene (G1691A) mutation, APC ratio;
prothrombin gene (G20210A) mutation;
methylentetra-hydrofolate reductase C677T mutation;
paroxysmal nocturnal hemoglobinuria;
transglutaminase antibodies;
Behcets disease clinical investigation;
- myeloproliferative disorders:
- JAK2 V617F mutation;
- Calreticulin exon 9 mutation;
- bone marrow biopsy;
- antiphospholipid syndrome:
- anticardiolipin antibodies;
- anti-beta 2GP1;
- lupus anticoagulant.
In more than two-thirds of cirrhotic patients with PVT, inherited thrombophilic disorders can be detected. The prothrombin gene 20210A mutation was found to be associated with a more than five-fold increased risk of developing PVT [18]. Additionally, factor-V-Leiden mutation and methylentetra-hydrofolate reductase C677T mutation are more frequently detected in cirrhotic patients with PVT compared to those without PVT [26]. However, in more recent studies, the causal relationship of these underlying thrombophilic disorders in the pathogenesis of PVT is controversially discussed [2].
Portal hemodynamics play a critical role in the pathogenesis of PVT in cirrhosis. Reduced portal blood flow results in an increased intrahepatic vascular resistance due to cirrhotic changes of the liver parenchyma and altered vascular reactivity. The reduction in portal blood flow velocity to less than 15 cm/sec on Doppler ultrasound has been identified as a significant predictive factor of PVT [27]. The use of non-selective beta-blockers may also contribute to the development of PVT by further reduction in portal blood flow [28].
Additionally, the development of portosystemic collaterals seems to negatively affect hemodynamic parameters in the portal vein: flow volume of more than 400 ml/min and blood flow velocity of more than 10 cm/sec in the largest collateral vessel have been identified to correlate with the occurrence of PVT [29].
The development of PVT in cirrhotic patients seems to be related to the severity of the disease, as it is more frequently detected in advanced stages [18]. In a recent study, however, the development of PVT in compensated cirrhosis was not directly related to cirrhosis progression, nor did PVT itself seem to aggravate the disease [30].
Vascular endothelial dysfunction may play a role in the pathogenesis of PVT. There is evidence indicating that, in patients with cirrhosis, markers of endothelial dysfunction, including von Willebrand factor, p-selectin and isoprostanes, are up-regulated [31], suggesting that endothelial cells in cirrhosis may favor the formation of thrombosis in the portal vein.
In addition to cirrhosis, neoplastic invasion of the portal vein is a major cause of PVT. Ögren et al. identified cancer as the cause of PVT in two-thirds of cirrhotic patients [3]. In these patients, PVT was related to invasion by primary hepatic cancer in one-third of cases with a prevalence of 14%. Thus, there appears to be a risk of developing PVT due to cirrhosis as well as hepatic cancer.
Inflammatory conditions, e.g. as a result of abdominal infections (spontaneous bacterial peritonitis, pancreatitis, cholecystitis, appendicitis, diverticulitis, etc.), have also been shown to be associated with the development of PVT [32]. It is plausible, although not yet demonstrated, that infectious conditions can sustain pro-thrombotic states and therefore should be considered in the management of PVT in cirrhotic patients.
The influence of the etiology of cirrhosis on the development of PVT is as yet insufficiently studied. However, neither viral hepatitis B or C nor alcohol abuse was found to be predictive of PVT. Intriguingly, in patients evaluated for liver transplantation, PVT was detected, in particular when cirrhosis was related to nonalcoholic steatohepatitis [5]. Further data are, however, needed to confirm these findings.
Primary prophylaxis
The concept of prophylactic treatment of PVT in cirrhosis was substantiated by a study showing that prophylactic doses of the low-molecular-weight heparin (LMWH) enoxaparin not only prevents PVT without increasing the rate of bleeding in cirrhotic patients, but also reduces bacterial translocation and liver decompensation [33]. It has been suggested that the coagulation cascade influences hepatic fibrogenesis via microthrombi and tissue ischemia, which activate the hepatic stellate cells [34], while enoxaparin reduced hepatic vascular resistance in experimental models of cirrhosis by prevention of hepatic stellate cell activation and decreased fibrogenesis [35]. Therefore, these data strongly suggest that anticoagulation could have a significantly more relevant impact not only on prevention of PVT, but also on slowing down liver fibrosis. These clinical observations deserve further investigation and advocate for primary prophylaxis using anticoagulants in cirrhotic patients.
As highlighted before, cirrhotic patients are not anticoagulated by default as suggested by their increased international normalized ratio. Therefore, thromboprophylaxis of cirrhotic patients for prevention of deep vein thrombosis is indicated similarly to recommendations in non-cirrhotic patients [36]. To date, this concept has not yet been included in international guidelines due to lack of randomized controlled trials (RCTs) [15]. However, some of the available data suggest that prophylactic PVT treatment may be indicated in cirrhotic patients awaiting liver transplantation or after hepatic resection [37,38].
Management
Aims and rationale
Therapy algorithms in current guidelines are based more on expert opinion than on results from RCTs [7]. The main goal of treatment is control and/or prevention of complications of PVT, which are accomplished by restoring the portal blood flow and prevention of thrombus extension. The latter is especially important, since thrombosis of the superior mesenteric vein might cause serious complications including mesenteric ischemia, which, in the setting of decompensated cirrhosis, could be associated with extremely high mortality. However, while some studies report that up to 70% of cases develop complete obstruction and/or extension to other splanchnic vessels [11,17,29,39], others report PVT mostly to be non-occlusive, associated with a high rate of spontaneous recanalization and without any influence on the clinical outcome [2]. However, patients with PVT have an increased risk of failure to control variceal bleeding and impaired survival after liver transplantation [39,40]. Therefore, the rationale to treat and achieve early patency is controversially discussed and the data published to date support different opinions. In summary, recanalization of the portal vein is especially important for liver-transplantation candidates, as end-to-end portal vein anastomosis is associated with better outcome after liver transplantation compared to other surgical techniques of portal vein reconstructions during liver transplantation [41]. Moreover, in patients with inflammatory conditions and in those at high risk of developing mesenteric ischemia, more aggressive approaches might be indicated. While studies show recurrent thrombosis after successful anticoagulation in almost 40% of cases after the anticoagulant is withdrawn [42], thrombus extinction occurs after transjugular intrahepatic portosystemic shunt (TIPS) [43], and the TIPS procedure might be a feasible secondary prophylaxis for PVT. However, data on this issue are scarce and prospective studies are needed [44]. Nevertheless, we suggest a step-wise escalation of treatment options, on which we will elaborate below [7,15].
Anticoagulation
Patient selection for anticoagulation remains controversial. According to the Baveno VI consensus, anticoagulation should be considered in potential liver-transplantation candidates with thrombosis of the main portal trunk or progressive PVT, with the goal to facilitate liver transplantation and reduce post-transplant morbidity and mortality [15]. For non-transplant candidates, no consensus has been found yet.
The data on safety and efficacy of anticoagulation are mainly based on five studies including a total of 176 patients, in most cases with partial PVT [37,39,42,45,46]. However, the proportion of partial, occlusive, extensive and cavernomatous PVT varied between the studies [44]. Under treatment, 8–50% of patients showed complete thrombus resolution, while partial resolution was observed in 33–45%. Thrombus progression was found in less than 10%. Late initiation of anticoagulation after onset of PVT (>6 months) correlated with recanalization failure [42]. Most patients had a history of bleeding or high-risk varices and received medical or endoscopic treatment prior to anticoagulation. In a study in which LMWHs were administered, no bleedings were observed [46]. However, another study reported bleedings in 20% of patients, particularly variceal bleedings in 9% [42]. In all five studies combined, bleeding episodes occurred in 5% [47]. Prolongation of treatment beyond 6 months was associated with lower thrombus progression and recurrence rate as well as a higher rate of recanalization [47].
Vitamin K antagonists (VKAs), LMWHs as well as direct-acting oral anticoagulants (DOACs) are currently available for the treatment of PVT. Data on LMWH and VKA demonstrated their efficacy and safety [37,39,42,45,46]. However, long-term effects in cirrhotic patients have not yet been systematically evaluated. Nonetheless, VKA and LMWH bear some caveats. While one advantage of VKA is its oral administration, associated with higher patient compliance, this leads to overestimation of the Model for End Stage Liver Disease (MELD) score, which is important for organ allocation in several systems (e.g. Eurotransplant). In patients with bleeding episodes, a platelet count <50 × 109/L and the use of VKA were the only factors more frequently observed and suspected to be anticoagulation-related [42].
Besides the beneficial pleiotropic effects of LMWH, subcutaneous administration might be a limitation, especially for long-term use [47]. However, their effect minimally interferes with the MELD score, while monitoring may be problematic due to low antithrombin levels and difficult measurement conditions for anti-factor-Xa in cirrhotic patients. Moreover, the accumulation of LMWH in renal dysfunction, a frequent problem in decompensated cirrhosis, may occur and lead to over-anticoagulation [46,48]. Importantly, enoxaparin has been studied in different doses (1.5 mg/kg vs 1.0 mg/kg per day) with similar efficacy, but lower bleeding rates at lower dose [49], suggesting that a dose adjustment in patients with cirrhosis might be indicated.
DOACs represent an attractive alternative to VKAs and LMWHs due to their oral administration and the fact that no regular laboratory monitoring is required. Moreover, first data show that use of DOAC is safe in cirrhotic patients [50,51]. However, the experience with DOACs is still limited and a strong recommendation cannot be made until more studies have become available.
In any case, the main concern when giving anticoagulants to cirrhotic patients is the risk of inducing or aggravating a bleeding episode, especially from varices. Screening endoscopy and standard prophylaxis (primary or secondary) of variceal bleeding, as recommended by the Baveno guidelines, are necessary prior to initiation of anticoagulation [15,49]. Importantly, no increase in 5-day treatment failure or 6-week mortality was reported in patients with upper GI bleeding under anticoagulation [52]. Adverse events were oral bleeding, lower GI bleeding, cerebral hemorrhage, vaginal bleeding, obscure GI bleeding and surgical wound hemorrhage. No anticoagulation-related deaths have been reported to date [37,42,45].
Interventional techniques
The endovascular approach in the management of PVT in cirrhotic patients aims at quickly restoring portal vein patency. A plethora of techniques and access routes have been published [7]. The most common and studied approach is the TIPS with local thrombus aspiration and thrombolysis. The reported success rates on catheter-directed thrombolysis are excellent. However, there are only limited data and a lack of RCTs considering this approach [53]. Besides aiming at restoring portal vein patency by direct catheter-directed lysis and removal of thrombus material, TIPS also decompresses the portal system by decreasing portal pressure and increasing portal flow velocity [54]. While the presence of PVT has historically been considered a contraindication for TIPS, technical improvements and increased expertise in experienced centers does not support this attitude. Indeed, in select cases, TIPS insertion can be successfully performed in patients with PVT [43]. In case of cavernomatous transformation, the technical procedure is more challenging with a successful TIPS placement rate of 62–74% [55,56]. Once TIPS can be placed, the recanalization rate is excellent (up to 80% partially or completely restored patency) [43]. This effect is possibly independent of anticoagulation [43,57]. In one study, residual thrombus was present in 77% of cases after TIPS placement, while, 1 month after the TIPS procedure, 76% of cases had complete resolution of thrombus. This suggests clot resolution due to increased portal venous flow. As these success rates are similar to those of anticoagulation, TIPS may provide an alternative for patients with contraindications for anticoagulation, severe symptoms or decompensation due to bleeding or ascites [47]. Other authors suggest TIPS in cases of early non-response to anticoagulation, extended/occlusive thrombus with high-risk varices or when symptomatic portal hypertension is present with PVT [44]. Special attention should be given to liver-transplantation candidates as a higher rate of surgical complications by misplaced TIPS stent has been described [58]. The shortest possible distance into the portal vein and no extension of the stent into the inferior vena cava (IVC) should be achieved. However, in most studies, the outcome was not impacted [59–62]. In contrast, the largest study to date showed improved graft and patient survival in TIPS patients [61].
Surgical approach
The primary care consisting of medical and interventional approaches for PVT as well as portal hypertension in general has led to a steady decrease in surgical interventions in these patients, resulting in a consequent decline in expertise in portal-caval shunt surgery [63]. However, emergency surgical intervention is still needed in cases of mesenteric ischemia and refractory variceal bleeding when the TIPS procedure is not feasible [7,15].
The surgical shunting procedures can be divided into three groups: total shunt (portal-caval end-to-side; splenorenal side-to-side (Cooley)), partial shunt (portal-caval side-to-side interposition (Sarfeh); mesocaval side-to-side interposition (Drapanas); splenorenal proximal end-to-side (Linton)) and selective shunt (mesorenal side-to-side; splenorenal distal end-to-side (Warren); coronary-caval (Inokuchi)) (Table 2). The choice of shunt procedure depends on liver function and the extent of PVT. Usually, operative decompression should be performed in patients with preserved liver function (Child-Pugh A and compensated Child-Pugh B) due to high rates of mortality (>50%) and hepatic encephalopathy in Child-Pugh C stage [15,64]. Generally, partial and selective shunts have replaced the total shunt due to its high rate of hepatic encephalopathy [65].
Table 2.
Options for surgical shunt procedures
| Total shunts | Partial shunts | Selective shunts | |
|---|---|---|---|
| Portocaval | End-to-side | Side-to-side interposition (Sarfeh) | |
| Mesocaval | Side-to-side interposition (Drapanas) | ||
| Mesorenal | Side-to-side | ||
| Splenorenal | Side-to-side (Cooley) | Proximal end-to-side (Linton) | Distal end-to-side (Warren) |
| Coronary-caval | Inokuchi |
Prognosis
The effect of PVT on patients’ survival after liver transplantation is still controversial [66–68]. The extent of PVT might be crucial to the post-liver-transplantation mortality [69,70]. Extensive PVT has been shown to be associated with higher mortality after liver transplantation, while incidentally discovered PVT during liver transplantation did not affect mortality [67,70]. However, studies found that complete PVT impacts short-term survival (3 months to 1 year), whereas long-term survival does not differ between PVT and non-PVT groups [41,71,72]. This effect might be due to the surgical techniques available for portal anastomosis depending on the extent of PVT. Survival rate in patients with non-anatomic anastomosis (cavoportal hemitransposition, renoportal anastomosis, portal vein arterialization) decreased, while in patients with physiologic anastomosis, survival is not impacted [41,73].
A study including 66,506 patients from the United Network for Organ Sharing registry (UNOS) showed that liver-transplantation waitin-list patients with PVT have lower mortality than those without PVT [74]. Other recent studies using data from the Scientific Registry of Transplant Recipients and Organ Procurement and Transplantation Network (OPTN) (n^^22,291) and single-center experience reported that presence of PVT does not increase mortality in waiting-list patients [66,71,75].
Conclusions and future perspectives
PVT in liver cirrhosis is an important clinical evolution, which could be an indication for or the cause of decompensation. Taking into account the general benefits of anticoagulation with LMWHs in cirrhotic patients, this treatment might be provided more regularly and to many patients, even to those without PVT but at risk of developing PVT. This, together with the role of DOACs in PVT, must be validated in the future. However, anticoagulation alone may not be sufficient to successfully treat cirrhotic patients with PVT. Especially in some patients with higher risk of developing complications and/or in candidates for liver transplantation, a more aggressive treatment should be considered, including interventional radiology. Overall, a significant amount of work remains to be done to improve the understanding and treatment of PVT and its underlying causes and consequences.
Acknowledgements
We thank Sabine Dentler for English proofreading and editing. Andrea De Gottardi is supported by the Swiss National Science Foundation (Grant 31003A_163143). Jonel Trebicka is supported by DFG (SFB TRR 57, P18) and Cellex-Foundation.
Conflict of interest statement: none declared.
References
- 1. Tsochatzis EA, Senzolo M, Germani G. et al. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther 2010;31:366–74. [DOI] [PubMed] [Google Scholar]
- 2. Nery F, Chevret S, Condat B. et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatol Baltim Md 2015;61:660–7. [DOI] [PubMed] [Google Scholar]
- 3. Ögren M, Bergqvist D, Björck M. et al. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23 796 consecutive autopsies. World J Gastroenterol 2006;12:2115–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chawla YK, Bodh V.. Portal vein thrombosis. J Clin Exp Hepatol 2015;5:22–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stine JG, Shah NL, Argo CK. et al. Increased risk of portal vein thrombosis in patients with cirrhosis due to nonalcoholic steatohepatitis. Liver Transpl 2015;21:1016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harding DJ, Perera MT, Chen F. et al. Portal vein thrombosis in cirrhosis: controversies and latest developments. World J Gastroenterol 2015;21:6769–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: vascular diseases of the liver. J Hepatol 2016;64:179–202. [DOI] [PubMed] [Google Scholar]
- 8. DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study Liver Diseases. Vascular disorders of the liver. Hepatol Baltim Md 2009;49:1729–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarin SK, Philips CA, Kamath PS. et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016;151:574–7.e3. [DOI] [PubMed] [Google Scholar]
- 10. Ghabril M, Agarwal S, Lacerda M. et al. Portal vein thrombosis is a risk factor for poor early outcomes after liver transplantation: analysis of risk factors and outcomes for portal vein thrombosis in waitlisted patients. Transplantation 2016;100:126–33. [DOI] [PubMed] [Google Scholar]
- 11. Englesbe MJ, Schaubel DE, Cai S. et al. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl 2010;16:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yerdel MA, Gunson B, Mirza D. et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation 2000;69:1873–81. [DOI] [PubMed] [Google Scholar]
- 13. Ma J, Yan Z, Luo J. et al. Rational classification of portal vein thrombosis and its clinical significance. PLoS One 2014;9:e112501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi J, Lai EC, Li N. et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci 2011;18:74–80. [DOI] [PubMed] [Google Scholar]
- 15. de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743–52. [DOI] [PubMed] [Google Scholar]
- 16. Malkowski P, Pawlak J, Michalowicz B. et al. Thrombolytic treatment of portal thrombosis. Hepatogastroenterology 2003;50:2098–100. [PubMed] [Google Scholar]
- 17. Sogaard KK, Astrup LB, Vilstrup H. et al. Portal vein thrombosis; risk factors, clinical presentation and treatment. BMC Gastroenterol 2007;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amitrano L, Guardascione MA, Brancaccio V. et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol 2004;40:736–41. [DOI] [PubMed] [Google Scholar]
- 19. Luca A, Caruso S, Milazzo M. et al. Natural course of extrahepatic nonmalignant partial portal vein thrombosis in patients with cirrhosis. Radiology 2012;265:124–32. [DOI] [PubMed] [Google Scholar]
- 20. Borjas-Almaguer OD, Cortez-Hernández CA, González-Moreno EI. et al. Portal vein thrombosis in patients with cirrhosis: just a common finding or a predictor of poor outcome? Ann Hepatol 2016;15:902–6. [DOI] [PubMed] [Google Scholar]
- 21. Rossi S, Rosa L, Ravetta V. et al. Contrast-enhanced versus conventional and color Doppler sonography for the detection of thrombosis of the portal and hepatic venous systems. AJR Am J Roentgenol 2006;186:763–73. [DOI] [PubMed] [Google Scholar]
- 22. Tublin ME, Dodd GD, Baron RL.. Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR Am J Roentgenol 1997;168:719–23. [DOI] [PubMed] [Google Scholar]
- 23. Tarantino L, Francica G, Sordelli I. et al. Diagnosis of benign and malignant portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma: color Doppler US, contrast-enhanced US, and fine-needle biopsy. Abdom Imaging 2006;31:537–44. [DOI] [PubMed] [Google Scholar]
- 24. Tripodi A, Primignani M, Mannucci PM. et al. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol 2016; 1 Nov. [DOI] [PubMed] [Google Scholar]
- 25. Lisman T, Porte RJ.. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood 2010;116:878–85. [DOI] [PubMed] [Google Scholar]
- 26. Amitrano L, Brancaccio V, Guardascione MA. et al. Inherited coagulation disorders in cirrhotic patients with portal vein thrombosis. Hepatol Baltim Md 2000;31:345–8. [DOI] [PubMed] [Google Scholar]
- 27. Zocco MA, Di Stasio E, De Cristofaro R. et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol 2009;51:682–9. [DOI] [PubMed] [Google Scholar]
- 28. Qi XS, Bai M, Fan DM.. Nonselective β-blockers may induce development of portal vein thrombosis in cirrhosis. World J Gastroenterol 2014;20:11463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maruyama H, Okugawa H, Takahashi M. et al. De novo portal vein thrombosis in virus-related cirrhosis: predictive factors and long-term outcomes. Am J Gastroenterol 2013;108:568–74. [DOI] [PubMed] [Google Scholar]
- 30. Complications of cirrhosis. Hepatology 2013;58:271A–4A. [Google Scholar]
- 31. La Mura V, Reverter JC, Flores-Arroyo A. et al. Von Willebrand factor levels predict clinical outcome in patients with cirrhosis and portal hypertension. Gut 2011;60:1133–8. [DOI] [PubMed] [Google Scholar]
- 32. Trebicka J, Strassburg CP.. Etiology and complications of portal vein thrombosis. Viszeralmedizin 2014;30:375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Villa E, Cammà C, Marietta M. et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012;143:1253–60.e1-4. [DOI] [PubMed] [Google Scholar]
- 34. Anstee QM, Dhar A, Thursz MR.. The role of hypercoagulability in liver fibrogenesis. Clin Res Hepatol Gastroenterol 2011;35:526–33. [DOI] [PubMed] [Google Scholar]
- 35. Cerini F, Vilaseca M, Lafoz E. et al. Enoxaparin reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol 2016;64:834–42. [DOI] [PubMed] [Google Scholar]
- 36. Aldawood A, Arabi Y, Aljumah A. et al. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J 2011;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Senzolo M, M Sartori T, Rossetto V. et al. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int 2012;32:919–27. [DOI] [PubMed] [Google Scholar]
- 38. Yamashita Y, Bekki Y, Imai D. et al. Efficacy of postoperative anticoagulation therapy with enoxaparin for portal vein thrombosis after hepatic resection in patients with liver cancer. Thromb Res 2014;134:826–31. [DOI] [PubMed] [Google Scholar]
- 39. Francoz C, Belghiti J, Vilgrain V. et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005;54:691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. post-therapeutic outcome and prognostic indicators. Hepatol Baltim Md 2003;38:599–612. [DOI] [PubMed] [Google Scholar]
- 41. Francoz C, Valla D, Durand F.. Portal vein thrombosis, cirrhosis, and liver transplantation. J Hepatol 2012;57:203–12. [DOI] [PubMed] [Google Scholar]
- 42. Delgado MG, Seijo S, Yepes I. et al. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol 2012;10:776–83. [DOI] [PubMed] [Google Scholar]
- 43. Luca A, Miraglia R, Caruso S. et al. Short- and long-term effects of the transjugular intrahepatic portosystemic shunt on portal vein thrombosis in patients with cirrhosis. Gut 2011;60:846–52. [DOI] [PubMed] [Google Scholar]
- 44. Rössle M, Bausch B, Klinger C.. Therapy algorithm for portal vein thrombosis in liver cirrhosis: the internist’s point of view. Viszeralmedizin 2014;30:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Werner KT, Sando S, Carey EJ. et al. Portal vein thrombosis in patients with end stage liver disease awaiting liver transplantation: outcome of anticoagulation. Dig Dis Sci 2013;58:1776–80. [DOI] [PubMed] [Google Scholar]
- 46. Amitrano L, Guardascione MA, Menchise A. et al. Safety and efficacy of anticoagulation therapy with low molecular weight heparin for portal vein thrombosis in patients with liver cirrhosis. J Clin Gastroenterol 2010;44:448–51. [DOI] [PubMed] [Google Scholar]
- 47. Primignani M, Tosetti G, La Mura V.. Therapeutic and clinical aspects of portal vein thrombosis in patients with cirrhosis. World J Hepatol 2015;7:2906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Potze W, Arshad F, Adelmeijer J. et al. Routine coagulation assays underestimate levels of antithrombin-dependent drugs but not of direct anticoagulant drugs in plasma from patients with cirrhosis. Br J Haematol 2013;163:666–73. [DOI] [PubMed] [Google Scholar]
- 49. Cui S, Shu R, Yan S. et al. Efficacy and safety of anticoagulation therapy with different doses of enoxaparin for portal vein thrombosis in cirrhotic patients with hepatitis B. Eur J Gastroenterol Hepatol 2015;27:914–19. [DOI] [PubMed] [Google Scholar]
- 50. De Gottardi A, Trebicka J, Klinger C. et al. Antithrombotic treatment with direct-acting oral anticoagulants in patients with splanchnic vein thrombosis and cirrhosis. Liver Int 2016; 25 Oct. [DOI] [PubMed] [Google Scholar]
- 51. Intagliata NM, Henry ZH, Maitland H. et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci 2016;61:1721–7. [DOI] [PubMed] [Google Scholar]
- 52. Cerini F, Gonzalez JM, Torres F. et al. Impact of anticoagulation on upper-gastrointestinal bleeding in cirrhosis: a retrospective multicenter study. Hepatol Baltim Md 2015;62:575–83. [DOI] [PubMed] [Google Scholar]
- 53. Schultheiß M, Bettinger D, Thimme R.. Nonsurgical therapeutic options in portal vein thrombosis. Viszeralmedizin 2014;30:388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rössle M. TIPS: 25 years later. J Hepatol 2013;59:1081–93. [DOI] [PubMed] [Google Scholar]
- 55. Senzolo M, Tibbals J, Cholongitas E. et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with and without cavernous transformation. Aliment Pharmacol Ther 2006;23:767–75. [DOI] [PubMed] [Google Scholar]
- 56. Han G, Qi X, He C. et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J Hepatol 2011;54:78–88. [DOI] [PubMed] [Google Scholar]
- 57. Salem R, Vouche M, Baker T. et al. Pretransplant portal vein recanalization-transjugular intrahepatic portosystemic shunt in patients with complete obliterative portal vein thrombosis. Transplantation 2015;99:2347–55. [DOI] [PubMed] [Google Scholar]
- 58. Clavien PA, Selzner M, Tuttle-Newhall JE. et al. Liver transplantation complicated by misplaced TIPS in the portal vein. Ann Surg 1998;227:440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. D’Avola D, Bilbao JI, Zozaya G. et al. Efficacy of transjugular intrahepatic portosystemic shunt to prevent total portal vein thrombosis in cirrhotic patients awaiting for liver transplantation. Transplant Proc 2012;44:2603–5. [DOI] [PubMed] [Google Scholar]
- 60. Tripathi D, Therapondos G, Redhead DN. et al. Transjugular intrahepatic portosystemic stent-shunt and its effects on orthotopic liver transplantation. Eur J Gastroenterol Hepatol 2002;14:827–32. [DOI] [PubMed] [Google Scholar]
- 61. Guerrini GP, Pleguezuelo M, Maimone S. et al. Impact of tips preliver transplantation for the outcome posttransplantation. Am J Transplant 2009;9:192–200. [DOI] [PubMed] [Google Scholar]
- 62. Barbier L, Hardwigsen J, Borentain P. et al. Impact of transjugular intrahepatic portosystemic shunting on liver transplantation: 12-year single-center experience. Clin Res Hepatol Gastroenterol 2014;38:155–63. [DOI] [PubMed] [Google Scholar]
- 63. Puhl G, Gül S, Neuhaus P.. Portosystemic shunt surgery between TIPS and liver transplantation. Chirurg 2011;82: 898–905. [DOI] [PubMed] [Google Scholar]
- 64. Wolff M, Hirner A.. Surgical treatment of portal hypertension. Zentralbl Chir 2005;130:238–45. [DOI] [PubMed] [Google Scholar]
- 65. Rosemurgy AS, Zervos EE.. Management of variceal hemorrhage. Curr Probl Surg 2003;40:263–343. [DOI] [PubMed] [Google Scholar]
- 66. Chen H, Turon F, Hernández-Gea V. et al. Nontumoral portal vein thrombosis in patients awaiting liver transplantation. Liver Transplant 2016;22:352–65. [DOI] [PubMed] [Google Scholar]
- 67. Rodríguez-Castro KI, Porte RJ, Nadal E. et al. Management of nonneoplastic portal vein thrombosis in the setting of liver transplantation: a systematic review. Transplantation 2012;94:1145–53. [DOI] [PubMed] [Google Scholar]
- 68. Ponziani FR, Zocco MA, Senzolo M. et al. Portal vein thrombosis and liver transplantation: implications for waiting list period, surgical approach, early and late follow-up. Transplant Rev Orlando Fla 2014;28:92–101. [DOI] [PubMed] [Google Scholar]
- 69. Fouzas I, Paul A, Becker C. et al. Orthotopic liver transplantation in patients with portal vein thrombosis in the absence of hepatocellular carcinoma. Transplant Proc 2012;44: 2734–6. [DOI] [PubMed] [Google Scholar]
- 70. Koh PS, Chan SC, Chok KS-H. et al. The friendly incidental portal vein thrombus in liver transplantation. Liver Transpl 2015;21:944–52. [DOI] [PubMed] [Google Scholar]
- 71. Englesbe MJ, Schaubel DE, Cai S. et al. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl 2010;16:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ghabril M, Agarwal S, Lacerda M. et al. Portal vein thrombosis is a risk factor for poor early outcomes after liver transplantation: analysis of risk factors and outcomes for portal vein thrombosis in waitlisted patients. Transplantation 2016;100:126–33. [DOI] [PubMed] [Google Scholar]
- 73. Hibi T, Nishida S, Levi DM. et al. When and why portal vein thrombosis matters in liver transplantation: a critical audit of 174 cases. Ann Surg 2014;259:760–6. [DOI] [PubMed] [Google Scholar]
- 74. Berry K, Taylor J, Liou IW. et al. Portal vein thrombosis is not associated with increased mortality among patients with cirrhosis. Clin Gastroenterol Hepatol 2015;13:585–93. [DOI] [PubMed] [Google Scholar]
- 75. John BV, Konjeti R, Aggarwal A. et al. Impact of untreated portal vein thrombosis on pre and post liver transplant outcomes in cirrhosis. Ann Hepatol 2013;12:952–8. [PubMed] [Google Scholar]