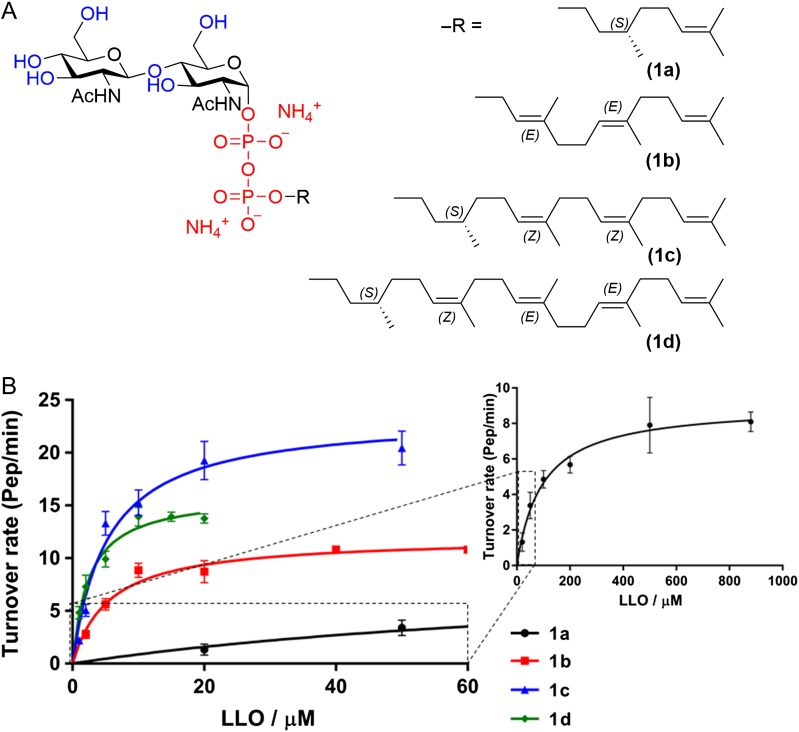
Fig. 3.
(A) Structure of the LLO analogs synthesized (1a) (S)-Citronellyl-PP-Chitobiose, C10; (1b) farnesyl-PP-chitobiose, C15; (1c) (S)-NerylCitronellyl-PP-chitobiose, C20; (1d) (S)-farnesylCitronellyl-PP-chitobiose, C25. (B) Kinetic analysis of synthetic LLO analogs. Glycosylation experiments were performed with 20 nM purified TbSTT3A, 10 µM peptide P14, 10 mM MnCl2, 150 mM NaCl, 20 mM Hepes pH 7.5, 0.035% DDM, 0.007% CHS and different concentrations of synthetic LLO analogs. Data points reflect the mean of 3 separate measurements. Error bars indicate standard deviations. Data were fitted by nonlinear regression according to the Michaelis–Menten formula using PRISM software. This figure is available in black and white in print and in color at Glycobiology online.