Abstract
Ascites is the most common complication related to cirrhosis and is associated with increased morbidity and mortality. Ascites is a consequence of the loss of compensatory mechanisms to maintain the overall effective arterial blood volume due to worsening splanchnic arterial vasodilation as a result of clinically significant portal hypertension. In order to maintain effective arterial blood volume, vasoconstrictor and antinatriuretic pathways are activated, which increase overall sodium and fluid retention. As a result of progressive splanchnic arterial vasodilation, intestinal capillary pressure increases and results in the formation of protein-poor fluid within the abdominal cavity due to increased capillary permeability from the hepatic sinusoidal hypertension. In some patients, the fluid can translocate across diaphragmatic fenestrations into the pleural space, leading to hepatic hydrothorax. In addition, infectious complications such as spontaneous bacterial peritonitis can occur. Eventually, as the liver disease progresses related to higher portal pressures, loss of a compensatory cardiac output and further splanchnic vasodilation, kidney function becomes compromised from worsening renal vasoconstriction as well as the development of impaired solute-free water excretion and severe sodium retention. These mechanisms then translate into significant clinical complications, such as refractory ascites, hepatorenal syndrome and hyponatremia, and all are linked to increased short-term mortality. Currently, liver transplantation is the only curative option for this spectrum of clinical manifestations but ongoing research has led to further insight on alternative approaches. This review will further explore the current understanding on the pathophysiology and management of ascites as well as expand on two advanced clinical consequences of advanced liver disease, refractory ascites and hyponatremia.
Keywords: ascites, portal hypertension, cirrhosis, hyponatremia
Introduction
Cirrhosis is the eighth leading cause of mortality in the United States [1] and is responsible for substantial annual direct and indirect costs exceeding $13 billion combined [2]. A large percentage of these costs are related to ascites, a complication of cirrhosis and portal hypertension that represents the most common liver-related reason for hospitalization [3]. Ascites has been associated with increased morbidity and mortality, with liver transplant-free mortality rates ranging from 15–20% in 1 year to nearly 50–60% in 5 years from the time of first onset [4–6]. Given the poor outcomes associated with ascites related to cirrhosis, it is critical to determine the candidacy for liver-transplantation evaluation initially in a patient’s assessment, as liver transplantation is the only curative option for cirrhosis and complications from portal hypertension. In addition, patients’ liver disease may further progress and develop refractory ascites (intolerant or nonresponsive to diuretic therapy), hepatorenal physiology or hyponatremia. The aim of this review is to expand on the current understanding, evaluation and management of ascites as well as the more advanced states of refractory ascites and hyponatremia.
Ascites
The pathophysiology of ascites
The development of increased intrahepatic resistance due to cirrhosis leads to a progressive increase in portal venous pressure. As the portal hypertension worsens, there is an increased local production of vasodilators, such as nitric oxide, due to endothelial activation and exposure to bacterial endotoxemia leading to splanchnic arterial vasodilation [7–9]. Compensatory mechanisms, such as antinatriuretic processes, sympathetic nervous system and renal vasoconstriction via the renin-angiotensin-aldosterone pathway, activate in order to maintain adequate effective arterial blood volume by increasing cardiac output as well as increasing overall plasma volume via renal sodium and fluid retention. However, as the liver disease progresses due to worsening portal hypertension and further vasodilation of the splanchnic arterial system, these compensatory mechanisms become ineffective and the effective arterial blood volume declines [10–12]. Additional sodium and fluid retention is attempted in order to maintain blood volume but, due to alterations in intestinal capillary pressure and permeability, as a net result of increased hydrostatic pressure and decreased oncotic pressure, free fluid accrues in the abdominal cavity [13]. Of patients with cirrhosis, 5–10% can present with a hepatic hydrothorax, ascitic fluid in the pleural space, presumed to be related to ascitic fluid translocating through diaphragmatic defects from the peritoneum to the pleural space [14].
Diagnosis, management and prognosis
Ascites can be assessed by proper physical examination as free fluid in the abdomen, although examination can be limited in the severely obese patient. Sonography can also easily assess for free fluid, either at the bedside or with a formal technician-performed ultrasound. Ascites is classified in three groups; in Grade 1, ascites fluid is detected only by ultrasound; in Grade 2, ascites is moderate with symmetrical distention of the abdomen; and, in Grade 3, ascites is large or tense, with marked abdominal distention. History and laboratory examination is critical to determine the etiology of the ascites, although over 90% of ascites cases are related to portal hypertension [15]. Diagnostic evaluation for cirrhosis and work-up of the ascitic fluid is important to perform at the time of first presentation as well as evaluating for possible complications such as spontaneous bacterial peritonitis (SBP) or renal failure. Diagnostic paracentesis is recommended by both the American Association for the Study of Liver Diseases (AASLD) and the European Association for the study of the Liver (EASL) at the time of first onset of ascites [16,17]. Historical questions and laboratory studies to perform on a patient with new-onset ascites are listed in Table 1. Classically, the serum–ascites albumin gradient is greater than or equal to 1.1 g/dL in the setting of portal hypertension [17].
Table 1.
The history/examination as well as diagnostic tests to work-up for patient with new-onset ascites
| Historical questions | Physical examination | Laboratory, fluid and imaging studies |
|---|---|---|
| Alcohol consumption? Current? | Vitals (blood pressure, heart rate) | Complete blood count, prothrombin time/ international normalized ratio |
| Sodium and dietary intake? | Jugular venous distension | Electrolyte and renal panel |
| Medications? NSAIDs? | Pulmonary exam | Liver function panel including albumin |
| Infections? | Cardiac exam (murmurs, irregular rate) | Urinalysis, urine electrolytes |
| Risk factors for viral hepatitis (blood transfusions, intravenous drug user, etc.) | Abdominal exam (free fluid, organomegaly) | Abdominal ultrasonography |
| Skin exam (jaundice, spider angiomata, palmar erythema) | Ascites fluid analysis (cell count with differential, fluid culture in blood culture bottles, albumin, total protein) | |
| Leg edema | ||
| Muscle wasting | ||
| Gynecomastia, testicular atrophy |
Once the etiology of the ascites has been determined to be a result of cirrhosis, the patient should be quickly evaluated on their candidacy for liver-transplantation evaluation, as the development of ascites is associated with poor survival [16]. Appropriate candidates should be immediately referred to a local liver-transplantation center so that early evaluation can be initiated regardless of the Model for End-stage Liver Disease (MELD) score at the time of referral. In addition, all patients with ascites should be educated on proper restrictions on sodium and, if they have concomitant hypervolemic hyponatremia, appropriate fluid restriction, as it is very common for patients to fail on proper dietary restrictions. The AASLD and EASL guidelines recommend restricting daily sodium intake to 2–4.6 grams in order to minimize worsening of fluid retention [16,17]. In addition, patients are advised to maintain an appropriate daily calorie intake of 25–40 kcal/kg and a protein intake of 1.2–1.5 g/kg, as ascites is a surrogate for protein-deficient malnourishment and always in the setting of hypoalbuminemia [18–20]. It is common for patients with large ascites to have early satiety as well as anorexia due to their liver condition and thus can further exacerbate their malnutrition state. Nutrition counseling with a dietician should always be considered.
Initiation of diuretics can be the first-line therapy to assist dietary restriction and promote an increase in volume removal. Guidelines recommend starting an aldosterone antagonist initially, often spironolactone, to assist with diuresis [16]. However, it is common to also use a loop diuretic in combination, usually furosemide, to enhance overall diuresis and lead to net fluid removal. Providers can evaluate for proper diuresis by testing the urine electrolytes to see whether the urine sodium has increased more than the urine potassium every 5–7 days. In addition, frequent weights can roughly determine proper diuresis, with both societies suggesting to aim for about 0.5 kilograms of fluid loss per day [21]. Serum electrolyte and renal function should be monitored in order to prevent over diuresis, electrolyte disturbances and possible pre-renal azotemia. Depending on the provider’s preference, the starting dose of spironolactone and furosemide typically has a daily dose ratio of 100 milligrams to 40 milligrams. Dose titration can be performed after 5–7 days based on urine electrolyte results, serum electrolyte and renal function, as well as clinical benefit with weight loss. Failure to lose fluid should initiate further questioning on that patient’s compliance with dietary restrictions with sodium, avoidance of alcohol, avoiding other medications such as non-steroidal anti-inflammatory drugs (NSAIDs) or the presence of an infection [16,17].
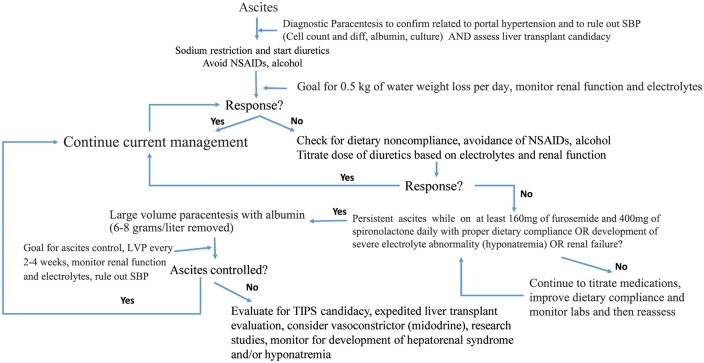
If dose titration of diuretics is unsuccessful and other causes are excluded, patients with larger-volume ascites or tense ascites (grade 2 and 3 ascites) may need intervention with a large-volume paracentesis (LVP). Per the AASLD guidelines, if removing more than 5 liters of ascites fluid, patients should receive additional volume expansion with intravenous albumin (6–8 grams per liter drained) in order to reduce the risk of circulatory dysfunction syndrome [16,22–24]. If the patient requires more than 2–3 LVPs in a month or cannot tolerate or respond to maximum dosed diuretics (160 mg/day of furosemide and 400 mg/day of spironolactone) with proper dietary compliance, then patients may have progressed to a more advanced stage of their liver disease, refractory ascites and thus, in some cases, may need to be assessed for transjugular intrahepatic portosystemic shunt placement (TIPS)—a placement of a nonsurgical shunt to immediately decompress portal hypertension, as either a bridge to liver transplantation or destination therapy [16]. We have attached a suggested algorithm for the management of ascites as shown in Figure 1.
Figure 1.
An algorithm for the management of ascites and refractory ascites
The development of ascites, as mentioned previously, is associated with significant decline in morbidity and mortality. The 1-year mortality risk for patients with cirrhosis increases from 1–3% to 15–20% after the development of ascites and as high as 50% in 5 years [5,25]. Liver transplantation is the only curative option to reverse the portal hypertension and remove the cirrhotic liver. In addition, increased mortality is often caused by infection, including SBP, pneumonia, urine infection or bloodstream infection, and any insult can quickly lead to acute renal dysfunction. There is growing evidence to consider TIPS to help treat select patients with ascites, regardless of transplant candidacy, as TIPS has been shown to be beneficial to decrease ascites volume, thus reducing the need for LVPs, decreasing the risk of SBP, and there is evidence for improved nutritional status and possible survival benefit [16,17,26]. In addition, patients who present with medically refractory hepatic hydrothorax should be considered for TIPS candidacy. If patients are deemed to not be TIPS candidates, serial LVPs with the appropriate dosing of intravenous albumin should be considered. If the patient is not a transplant candidate, consideration for destination TIPS or serial LVPs should be assessed as well as referral to palliative care services. Referral to palliative care for patients with advanced liver disease has been shown to objectively improve clinical and patient-reported outcomes and this service’s role in both transplant and non-transplant settings will need to be further explored [27,28]. Regardless, the development of ascites is a serious complication of advanced cirrhosis and portal hypertension, and reporting to the patient on their prognosis is critical.
SBP
Patients with ascites from cirrhosis can develop SBP, an acute infection of the ascitic fluid. Historically, it was reported that SBP occurred in 10–20% in patients admitted to the hospital with ascites; however, it is believed that this rate is lower now due to the incorporation of prophylaxis albeit the rate of antibiotic resistant organisms has increased [16,29]. It is essential for patients with ascites, whether new-onset, asymptomatic or presenting with clinical changes such as hypotension or acute kidney injury including hepatorenal syndrome, to have a diagnostic paracentesis to exclude SBP. Peritonitis requires early diagnosis and initiation of treatment, as any delay in therapy has been association with poor outcomes, including risk for hepatorenal syndrome and death [30,31]. SBP is a common reason for patients with cirrhosis, ascites and acute kidney injury (AKI) and can commonly lead to acute or chronic liver failure [32,33]. The diagnosis of SBP is made by diagnostic paracentesis and the ascitic fluid cell count consists of an elevated absolute polymorphonuclear leukocyte count ≥250 cells/mm3. Cultures in blood culture bottles should also be sent in order to capture an offending organism and provide directed antibiotics. In patients with SBP, therapy should consist of antibiotics (i.e. Ceftriaxone 2 grams every 24 hours) plus albumin (1.5 g/kg at the diagnosis and 1 g/kg every 48 hours), as combination therapy has been found to prevent renal impairment in these patients, although some recent studies may agree that the most benefit of albumin is seen in those with more severe organ impairment [34,35]. Specific recommendations for antibiotics can be reviewed on the AASLD or EASL Guidelines for Ascites and treatment of SBP [16,17].
Refractory ascites
The pathophysiology of refractory ascites
As liver disease progresses and the effective arterial blood volume declines, compensatory mechanisms involving the sympathetic nervous system, antinatriuretic factors and renal vasoconstriction attempt to improve blood volume by increasing plasma volume and increasing overall sodium and fluid retention. After loss of oncotic pressure from hypoalbuminemia and development of ascites from the increased intestinal capillary leakage, patients eventually lose the ability to maintain effective arterial blood volume. This, with time, leads to severe fluid retention from impaired renal solute-free water excretion and renal vasoconstriction [36]. These consequences from progressive liver disease lead to serious complications related to ascites, hypervolemic hyponatremia, refractory ascites or hepatorenal physiology. Hepatorenal syndrome will be addressed in more detail in another review. Refractory ascites refers to the inability to respond to medical and dietary management (whether intolerant or unresponsive to diuretics) or rapid reaccumulation of ascites after LVP [37,38]. Refractory ascites occurs in patients who have severe sodium and fluid retention and have lost their compensatory pathways, including inadequate cardiac output and inability to maintain appropriate effective arterial blood volume. Fortunately, refractory ascites only occurs in 10% of patients with cirrhosis and ascites [37,39,40].
Diagnosis, management and prognosis
As per the AASLD and EASL guidelines, refractory ascites is defined as ascites that is unresponsive to the appropriate sodium-restricted diet and high-dose diuretics (160 milligrams of daily furosemide and 400 milligrams of daily spironolactone). In addition, patients meet criteria if they have rapid reaccumulation of ascites after therapeutic paracentesis. Failure of diuretic therapy is defined as the inability to maintain adequate urinary sodium excretion (<78 mmol per day) or complications of diuretics including hepatic encephalopathy, renal failure or hyponatremia [16,17]. Therefore, assessment for dietary and medication compliance, alcohol abstinence and presence of infection needs to be performed prior to the diagnosis of refractory ascites. Refractory ascites is a clinical manifestation associated with higher short-term mortality rates and timely evaluation for liver transplantation and/or TIPS should be performed [16,41,42]. Liver transplantation remains the only curative option, but therapeutic options include use of maintenance midodrine therapy to help increase arterial blood pressure, serial LVPs with albumin or TIPS placement [16,43]. Initial control of refractory ascites is performed with serial LVPs every 2–4 weeks with intravenous albumin and can be performed in the outpatient setting. However, effects are short-lived, with most having early recurrence of ascites. Peritoneovenous shunts are available, but their use has declined in favor of TIPS due to associated complications related to the shunt [40,44]. Among older cohorts and trials that investigated the efficacy of TIPS in comparison to LVP and albumin, TIPS was associated with improved control of ascites; however, these analyses did not find patients with TIPS to have improved survival and had increased rates of hepatic encephalopathy [41,42,45–50]. Researchers felt the lack of survival benefit may have been related to the use of uncovered stents. Therefore, a more recent cohort of patients using only polytetrafluoroethylene-covered stents revealed that TIPS was a significantly more effective treatment option in appropriately selected patients, including improved transplant-free survival as well as ascites control without major detriment from hepatic encephalopathy [26]. In addition, the discontinuation of beta blockers for this patient population remains controversial and we would recommend that decisions to be performed on a case-by-case basis at this time [16]. We have attached our suggested treatment algorithm in Figure 1.
The prognosis of refractory ascites is very grim and thus expedited referral to a liver-transplantation center is critical for appropriate transplant candidates. Patients with refractory ascites have an increased risk for infection, especially SBP, as well as risk for further liver decompensation such as hepatic encephalopathy, variceal hemorrhage and risk of developing hepatorenal syndrome. The probability of developing refractory ascites is around 11% in 5 years. Patients with refractory ascites carry a 1-year mortality rate of near 70% and over 50% can lead to hepatorenal syndrome [40]. More prospective studies are needed to further elucidate the optimal treatment strategies for patients with refractory ascites.
Hyponatremia
The pathophysiology of hyponatremia
Hyponatremia in cirrhosis is defined as a serum sodium <130 mEq/L. This complication is another consequence of advanced portal hypertension, extreme sodium and free water retention, as well as the loss of compensatory mechanisms to maintain effective arterial blood volume, as discussed earlier in the review. More than half of hospitalized patients with ascites present with hyponatremia [51]. Hypovolemic hyponatremia refers to fluid losses from the kidney (iatrogenic overdiuresis) or from the gastrointestinal tract (diarrhea, particularly from lactulose). Serum sodium levels usually improve after resolution of the etiology and replacement of their plasma volume usually with fluid replacement. However, hypervolemic hyponatremia is more commonly seen in cirrhosis and relates to inappropriate impaired renal excretion of solute-free water in the setting of severe sodium and water retention [52–54]. In this setting, the antinatriuretic pathway involves the over-secretion of arginine vasopressin (AVP) that enhances the function of the vasopressin 2 (V2) receptors within the renal distal collecting tubules and inhibits solute-free water excretion [55]. In the setting of increased AVP production as well as lack of clearance of AVP due to cirrhosis, V2 is excessively bound by the AVP, triggering further free water retention in the renal tubules by forming more aquaporin-2 channels to retain more water [56,57]. Therefore, patients cannot remove enough water and this results in worsening serum dilution and hypo-osmolality [17,56].
Diagnosis, management and prognosis
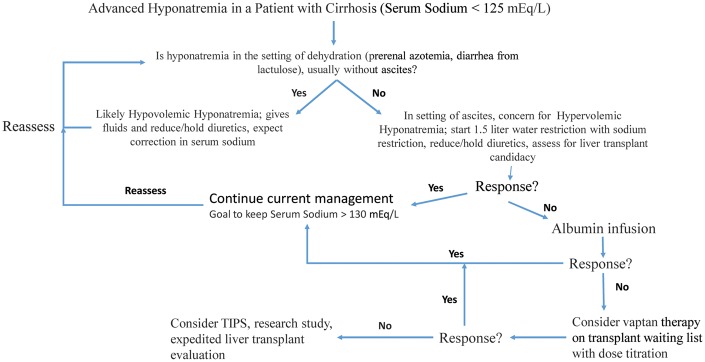
Diagnosis of hyponatremia in cirrhosis is simply based on laboratory work showing a decreased serum sodium level of under 130 mEq/L. If evidence of dehydration or pre-renal azotemia, treatment of the underlying cause and repletion of volume will improve hypovolemic hyponatremia [55]. However, in the setting of volume overload, hypervolemic hyponatremia is much more difficult for patients to tolerate and to reverse. The mainstay of therapy consists of water restriction and increasing the renal excretion of free water [17]. Dietary restriction of fluids to 1.5 liters daily is recommended, especially when the serum sodium is less than 130 mEq/L but patient compliance is poor and likelihood of response is low. Diuretic dose adjustments or even discontinuation may be required. Alternatively, increasing the effective arterial blood volume with intravenous albumin with or without vasoconstrictors, such as midodrine, shows promise in nonrandomized studies [58,59]. However, these therapies require further investigation prior to incorporation into practice. A promising therapeutic option for hyponatremia is to consider the use of a class of drugs called the ‘vaptans’, specific V2-receptor antagonists, that induce release of solute-free water into the urine and correct the hyponatremia in the setting of cirrhosis [60–65]. These drugs are very effective, correcting serum sodium levels in 45–82% of patients [17]. Thirst is a particular side effect to be wary of as well as concerns for dehydration, renal injury and overcorrection of sodium. However, the duration of response is short and reverts back to baseline hyponatremia after drug discontinuation. In addition, trials involving the vaptans did not demonstrate improvement in survival compared to the placebo arms [66,67]. Therefore, most experts will suggest using vaptans for short periods of time, such as candidates who are hospitalized awaiting their liver transplant and need their hyponatremia corrected prior to surgery in the setting of severe hyponatremia (<125 mEq/L) [17].
Hyponatremia can occur in up to 50% of patients with cirrhosis and ascites, and even up to 10–20% of patients with more advanced hyponatremia (sodium serum ≤125 mEq/L). The presence of hyponatremia has been associated with increased morbidity and mortality independently of other prognosticators and has been recently added to the MELD score (Sodium-MELD) for liver donor allocation in the United States [54,68–70]. For each drop in unit of sodium below 135 mEq/L, the mortality risk increases by over 10% for patients listed for liver transplant [71]. In addition, the presence of hyponatremia and its management are poorly tolerated and related to significant decline in quality of life as well as linked to worsening hepatic encephalopathy and increased neurological complications during and after liver-transplantation surgery, such as osmotic demyelination [71–75]. Most transplant centers will require correction of a patient’s hyponatremia prior to surgery, but this issue remains in debate and is not standardized across all programs. Our suggested algorithm for treatment of hyponatremia is provided in Figure 2.
Figure 2.
An algorithm for the management of hyponatremia
Future directions
Ascites and the clinical manifestations from worsening splanchnic arterial vasodilation and decreased effective arterial blood volume related to severe portal hypertension, including refractory ascites and hyponatremia, remain ominous conditions for patients with advanced cirrhosis and carry high short-term mortality rates as well as poor quality of life. As access to liver transplantation continues to decrease due to shortage of acceptable organs, strategies to effectively manage these patients in order to bridge them successfully to liver transplantation is critical and current therapies remain suboptimal and poorly tolerated. Developments of new drugs or interventions that can successfully act on the splanchnic arterial vasodilation or improve the effective arterial blood volume depletion are potential targets for further research in order to increase our knowledge and enhance clinical practice.
Further exploration into agents that successfully expand the effective arterial blood volume, such as albumin or another colloid agent, as well as increasing vasoconstriction with an agent, such as midodrine, demands high-quality, randomized–controlled trials. The small amount of existing data in the use of midodrine (and in other countries, terlipressin) have demonstrated improved renal function, increased urinary sodium excretion and decreased levels of vasodilatory and antinatriuretic systems in those with ascites [76]. One clinical study showed that oral midodrine in combination with dietary sodium restriction and oral diuretics had significantly improved ascites control and survival at 3 months compared to those without midodrine [43]. Terlipressin, a vasopressin analogue commonly used outside the United States for variceal hemorrhage and hepatorenal syndrome, has also been studied in ascites but these data are limited and the drug is currently not available in the United States. [77,78]. Further exploration on these agents as well as newer drugs in the pipeline that can ameliorate the systemic vasodilatory state and reduce the activation of the sympathetic nervous and renin-angiotensin-aldosterone systems are required in order to create enhanced treatment strategies for the management of ascites.
Another class of drugs, the ‘vaptans’, also provides a promising therapeutic option. Although these agents were mainly studied on patients with cirrhosis and hyponatremia, there are a few trials that investigated their use for ascites and refractory ascites. One vaptan, satavaptan, was used for the treatment of ascites but the results failed to show any benefit in ascites control and even was found to have increased mortality risk, leading to market removal [79,80]. In addition, tolvaptan, studied in refractory ascites, has been approved by the US Food and Drug Administration (FDA) for use in hyponatremia, but was found to have a high rate of hepatic toxicity (23%) and was assigned a black-box warning by the FDA for the drug’s use in patients with liver disease [81–84]. Further investigation and drug development in order to find efficacious targets that can safely reverse the pathophysiological mechanisms in cirrhotics’ severe sodium and water retention remain in great demand.
An automated low-flow pump system (Alfapump system) was recently introduced for the treatment of refractory ascites. It is a subcutaneous battery-operated pump that was designed to move ascites from peritoneum to the urinary bladder, where it is eliminated spontaneously through diuresis. Although designed as a symptomatic treatment for refractory ascites, it does not correct the underlying cause of the ascites that could help improve the pathogenic mechanisms that lead to the formation of ascites. Data are limited to small studies and, although the pump is effective in removing ascites in most patients, there are no differences in survival when compared to LVP and side effects are more common.
In addition, further exploration into pre-emptive TIPS or other timing strategies with portal decompression need future investigation in order to understand the reversibility of complications related to clinically significant portal hypertension as well as improving our selection of patients who receive more benefit instead of harm by enhanced risk stratification. Knowledge gaps definitely remain in the field and future investment as well as collaboration and coordination of experts are necessary in order to further advance our understanding of advanced liver disease and its complications.
Conclusions
Ascites remains the most common reason for hospitalization for patients with advanced cirrhosis and simply the initial presentation of ascites is associated with significant survival decline. In addition, ascites is responsible for significant healthcare spending, associated with poor quality of life, and opens a door to the ‘slippery slope’ of developing even further stages of decompensation with SBP, refractory ascites, hepatorenal syndrome, hyponatremia and eventually leading to death. Our understanding on the pathophysiology of ascites formation and the hemodynamic changes in the systemic and splanchnic vascular systems has dramatically matured over the last 40 years, but the field still demands more for further investigation and investment in order to translate our comprehension into improved outcomes. The presence of ascites still carries a poor prognosis without liver transplantation and, with access to liver transplantation becoming more difficult due to a decline in donor organs in the United States, the demand to improve our therapeutic options for ascites and other complications of portal hypertension has become essential.
We have learned that the development of a decreased effective arterial blood volume due to worsening splanchnic arterial vasodilation related to the local production of vasodilators (nitric oxide, endotoxins) is the basis of ascites formation and other cirrhotic complications. As research continues to investigate for new targets that are involved in these pathways, our field will hopefully make more strides in creating new effective agents. In addition, research networks and specialized coordination among institutions and societies, such as AASLD and EASL, will need to continue to build and collaborate together to develop new high-quality studies that can effectively investigate clinical questions as well as translate those results to bench and back again to the bedside.
Without liver transplant, there is no current cure for patients with advanced cirrhosis and ascites, and therefore timely referral to a liver-transplant center for evaluation is critical while potential strategies to help with portal decompression such as TIPS used in the correct population of patients may provide a bridge to transplant as well as destination therapy. Meanwhile, dietary sodium restriction is imperative to successfully remove volume while using a combination of diuretics in the majority of patients with ascites. Taking a good history is also important in order to look for any issues with patient compliance, ingestion of offending medications or consumption of alcohol. In advanced cases, such as refractory ascites, serial LVP with albumin and, in some patients, TIPS placement may need to be considered. In the presence of hypervolemic hyponatremia, patients are requested to restrict their sodium and fluid intake but tolerance due to excessive thirst limits this recommendation’s effectiveness. Additional services to help improve the decreased quality of care of these patients may open the door for opportunities to work with other healthcare models, such as a palliative care service, that may provide further patient benefit and incorporation of these care models should be further explored [85]. Ascites is a large healthcare cost burden and healthcare spending will continue to increase dramatically as cases related to non-alcoholic steatohepatitis and untreated viral hepatitis substantially grow in number. Newer strategies are needed to provide more effective treatment for ascites and its complications, as well as more sophisticated outpatient care models in order to improve clinical outcomes while reducing healthcare spending.
Conflict of interest statement: none declared.
References
- 1. Ge PS, Runyon BA.. Treatment of patients with cirrhosis. N Engl J Med 2016;375:767–77. [DOI] [PubMed] [Google Scholar]
- 2. Everhart JE, Ruhl CE.. Burden of digestive diseases in the United States. Part I: Overall and upper gastrointestinal diseases. Gastroenterology 2009;136:376–86. [DOI] [PubMed] [Google Scholar]
- 3. Volk ML, Tocco RS, Bazick J. et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012;107:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Planas R, Montoliu S, Balleste B. et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol 2006;4:1385–94. [DOI] [PubMed] [Google Scholar]
- 5. D’Amico G, Garcia-Tsao G, Pagliaro L.. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217–31. [DOI] [PubMed] [Google Scholar]
- 6. Gines P, Quintero E, Arroyo V. et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology 1987;7:122–8. [DOI] [PubMed] [Google Scholar]
- 7. Schrier RW, Arroyo V, Bernardi M. et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 1988;8:1151–7. [DOI] [PubMed] [Google Scholar]
- 8. Wiest R, Garcia-Tsao G.. Bacterial translocation (BT) in cirrhosis. Hepatology 2005;41:422–33. [DOI] [PubMed] [Google Scholar]
- 9. Iwakiri Y. Endothelial dysfunction in the regulation of cirrhosis and portal hypertension. Liver Int 2012;32:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schrier RW. Pathogenesis of sodium and water retention in high-output and low-output cardiac failure, nephrotic syndrome, cirrhosis, and pregnancy (2). N Engl J Med 1988;319:1127–34. [DOI] [PubMed] [Google Scholar]
- 11. Ruiz-del-Arbol L, Monescillo A, Arocena C. et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology 2005;42:439–47. [DOI] [PubMed] [Google Scholar]
- 12. Krag A, Bendtsen F, Henriksen JH. et al. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut 2010;59:105–10. [DOI] [PubMed] [Google Scholar]
- 13. Witte CL, Witte MH, Dumont AE.. Lymph imbalance in the genesis and perpetuation of the ascites syndrome in hepatic cirrhosis. Gastroenterology 1980;78:1059–68. [PubMed] [Google Scholar]
- 14. Lazaridis KN, Frank JW, Krowka MJ. et al. Hepatic hydrothorax: pathogenesis, diagnosis, and management. Am J Med 1999;107:262–7. [DOI] [PubMed] [Google Scholar]
- 15. Runyon BA, Montano AA, Akriviadis EA. et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992;117:215–20. [DOI] [PubMed] [Google Scholar]
- 16. Runyon BA., AASLD Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology 2013;57:1651–3. [DOI] [PubMed] [Google Scholar]
- 17. European Association for the Study of the L. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010;53:397–417. [DOI] [PubMed] [Google Scholar]
- 18. Swart GR, van den Berg JW, van Vuure JK. et al. Minimum protein requirements in liver cirrhosis determined by nitrogen balance measurements at three levels of protein intake. Clin Nutr 1989;8:329–36. [DOI] [PubMed] [Google Scholar]
- 19. Eghtesad S, Poustchi H, Malekzadeh R.. Malnutrition in liver cirrhosis:the influence of protein and sodium. Middle East J Dig Dis 2013;5:65–75. [PMC free article] [PubMed] [Google Scholar]
- 20. Henkel AS, Buchman AL.. Nutritional support in patients with chronic liver disease. Nat Clin Pract Gastroenterol Hepatol 2006;3:202–9. [DOI] [PubMed] [Google Scholar]
- 21. Pockros PJ, Reynolds TB.. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology 1986;90:1827–33. [DOI] [PubMed] [Google Scholar]
- 22. Salerno F, Badalamenti S, Incerti P. et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients: a safe alternative therapy. J Hepatol 1987;5:102–8. [DOI] [PubMed] [Google Scholar]
- 23. Gines P, Tito L, Arroyo V. et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology 1988;94:1493–1502. [DOI] [PubMed] [Google Scholar]
- 24. Ruiz-del-Arbol L, Monescillo A, Jimenez W. et al. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology 1997;113:579–86. [DOI] [PubMed] [Google Scholar]
- 25. Fattovich G, Giustina G, Degos F. et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 1997;112:463–72. [DOI] [PubMed] [Google Scholar]
- 26. Bureau C, Thabut D, Oberti F. et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017;152:157–63. [DOI] [PubMed] [Google Scholar]
- 27. Baumann AJ, Wheeler DS, James M. et al. Benefit of early palliative care intervention in end-stage liver disease patients awaiting liver transplantation. J Pain Symptom Manage 2015;50:882–6.e2. [DOI] [PubMed] [Google Scholar]
- 28. Larson AM. Palliative care for patients with end-stage liver disease. Curr Gastroenterol Rep 2015;17:440. [DOI] [PubMed] [Google Scholar]
- 29. Piano S, Fasolato S, Salinas F. et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: results of a randomized, controlled clinical trial. Hepatology 2016;63:1299–1309. [DOI] [PubMed] [Google Scholar]
- 30. Kim JJ, Tsukamoto MM, Mathur AK. et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol 2014;109:1436–42. [DOI] [PubMed] [Google Scholar]
- 31. Orman ES, Hayashi PH, Bataller R. et al. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol 2014;12: 496–503.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duseja A, Chawla YK, Dhiman RK. et al. Non-hepatic insults are common acute precipitants in patients with acute on chronic liver failure (ACLF). Dig Dis Sci 2010;55:3188–92. [DOI] [PubMed] [Google Scholar]
- 33. Bajaj JS, O’Leary JG, Reddy KR. et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014;60:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sort P, Navasa M, Arroyo V. et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 1999;341:403–9. [DOI] [PubMed] [Google Scholar]
- 35. Sigal SH, Stanca CM, Fernandez J. et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut 2007;56:597–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gines P, Cardenas A, Arroyo V. et al. Management of cirrhosis and ascites. N Engl J Med 2004;350:1646–54. [DOI] [PubMed] [Google Scholar]
- 37. Arroyo V, Gines P, Gerbes AL. et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis: International Ascites Club. Hepatology 1996;23:164–76. [DOI] [PubMed] [Google Scholar]
- 38. Salerno F, Guevara M, Bernardi M. et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int 2010;30:937–47. [DOI] [PubMed] [Google Scholar]
- 39. Perez-Ayuso RM, Arroyo V, Planas R. et al. Randomized comparative study of efficacy of furosemide versus spironolactone in nonazotemic cirrhosis with ascites: relationship between the diuretic response and the activity of the renin-aldosterone system. Gastroenterology 1983;84:961–8. [PubMed] [Google Scholar]
- 40. Stanley MM, Ochi S, Lee KK. et al. Peritoneovenous shunting as compared with medical treatment in patients with alcoholic cirrhosis and massive ascites. Veterans Administration Cooperative Study on Treatment of Alcoholic Cirrhosis with Ascites. N Engl J Med 1989;321:1632–8. [DOI] [PubMed] [Google Scholar]
- 41. Sanyal AJ, Genning C, Reddy KR. et al. The North American Study for the Treatment of Refractory Ascites. Gastroenterology 2003;124:634–41. [DOI] [PubMed] [Google Scholar]
- 42. Saab S, Nieto JM, Lewis SK. et al. TIPS versus paracentesis for cirrhotic patients with refractory ascites. Cochrane Database Syst Rev 2006:CD004889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh V, Dhungana SP, Singh B. et al. Midodrine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. J Hepatol 2012;56:348–54. [DOI] [PubMed] [Google Scholar]
- 44. Gines P, Arroyo V, Vargas V. et al. Paracentesis with intravenous infusion of albumin as compared with peritoneovenous shunting in cirrhosis with refractory ascites. N Engl J Med 1991;325:829–35. [DOI] [PubMed] [Google Scholar]
- 45. Rossle M, Ochs A, Gulberg V. et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med 2000;342:1701–7. [DOI] [PubMed] [Google Scholar]
- 46. Gines P, Uriz J, Calahorra B. et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology 2002;123: 1839–47. [DOI] [PubMed] [Google Scholar]
- 47. Salerno F, Merli M, Riggio O. et al. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology 2004;40:629–35. [DOI] [PubMed] [Google Scholar]
- 48. Deltenre P, Mathurin P, Dharancy S. et al. Transjugular intrahepatic portosystemic shunt in refractory ascites: a meta-analysis. Liver Int 2005;25:349–56. [DOI] [PubMed] [Google Scholar]
- 49. Salerno F, Camma C, Enea M. et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology 2007;133:825–34. [DOI] [PubMed] [Google Scholar]
- 50. Tan HK, James PD, Sniderman KW. et al. Long-term clinical outcome of patients with cirrhosis and refractory ascites treated with transjugular intrahepatic portosystemic shunt insertion. J Gastroenterol Hepatol 2015;30:389–95. [DOI] [PubMed] [Google Scholar]
- 51. Angeli P, Wong F, Watson H. et al. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology 2006;44:1535–42. [DOI] [PubMed] [Google Scholar]
- 52. Fortune BE, Garcia-Tsao G.. Hypervolemic hyponatremia: clinical significance and management. Clinical Liver Disease 2013;2:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gines P, Berl T, Bernardi M. et al. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology 1998;28:851–64. [DOI] [PubMed] [Google Scholar]
- 54. Arroyo V, Rodes J, Gutierrez-Lizarraga MA. et al. Prognostic value of spontaneous hyponatremia in cirrhosis with ascites. Am J Dig Dis 1976;21:249–56. [DOI] [PubMed] [Google Scholar]
- 55. Gines P, Guevara M.. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology 2008;48:1002–10. [DOI] [PubMed] [Google Scholar]
- 56. Lizaola B, Bonder A, Tapper EB. et al. The changing role of sodium management in cirrhosis. Curr Treat Options Gastroenterol 2016;14:274–84. [DOI] [PubMed] [Google Scholar]
- 57. Thibonnier M, Conarty DM, Preston JA. et al. Molecular pharmacology of human vasopressin receptors. Adv Exp Med Biol 1998;449:251–76. [DOI] [PubMed] [Google Scholar]
- 58. McCormick PA, Mistry P, Kaye G. et al. Intravenous albumin infusion is an effective therapy for hyponatraemia in cirrhotic patients with ascites. Gut 1990;31:204–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Singhal S, Baikati KK, Jabbour II. et al. Management of refractory ascites. Am J Ther 2012;19:121–32. [DOI] [PubMed] [Google Scholar]
- 60. Schrier RW, Gross P, Gheorghiade M. et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006;355:2099–2112. [DOI] [PubMed] [Google Scholar]
- 61. Cardenas A, Gines P, Marotta P. et al. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol 2012;56:571–8. [DOI] [PubMed] [Google Scholar]
- 62. Pose E, Solà E, Piano S. et al. Limited efficacy of tolvaptan in patients with cirrhosis and severe hyponatremia: real-life experience. Am J Med 2017;130:372–5. [DOI] [PubMed] [Google Scholar]
- 63. O’Leary JG, Davis GL.. Conivaptan increases serum sodium in hyponatremic patients with end-stage liver disease. Liver Transpl 2009;15:1325–9. [DOI] [PubMed] [Google Scholar]
- 64. Gerbes AL, Gulberg V, Gines P. et al. Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: a randomized double-blind multicenter trial. Gastroenterology 2003;124:933–9. [DOI] [PubMed] [Google Scholar]
- 65. Wong F, Blei AT, Blendis LM. et al. A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: a multicenter, randomized, placebo-controlled trial. Hepatology 2003;37:182–91. [DOI] [PubMed] [Google Scholar]
- 66. Yan L, Xie F, Lu J. et al. The treatment of vasopressin V2-receptor antagonists in cirrhosis patients with ascites: a meta-analysis of randomized controlled trials. BMC Gastroenterol 2015;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dahl E, Gluud LL, Kimer N. et al. Meta-analysis: the safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment Pharmacol Ther 2012;36:619–26. [DOI] [PubMed] [Google Scholar]
- 68. Biggins SW, Rodriguez HJ, Bacchetti P. et al. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology 2005;41:32–9. [DOI] [PubMed] [Google Scholar]
- 69. Ruf AE, Kremers WK, Chavez LL. et al. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl 2005;11:336–43. [DOI] [PubMed] [Google Scholar]
- 70. Londono MC, Cardenas A, Guevara M. et al. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut 2007;56:1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Londono MC, Guevara M, Rimola A. et al. Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation. Gastroenterology 2006;130:1135–43. [DOI] [PubMed] [Google Scholar]
- 72. Dawwas MF, Lewsey JD, Neuberger JM. et al. The impact of serum sodium concentration on mortality after liver transplantation: a cohort multicenter study. Liver Transpl 2007;13:1115–24. [DOI] [PubMed] [Google Scholar]
- 73. Yun BC, Kim WR, Benson JT. et al. Impact of pretransplant hyponatremia on outcome following liver transplantation. Hepatology 2009;49:1610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guevara M, Baccaro ME, Torre A. et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol 2009;104:1382–9. [DOI] [PubMed] [Google Scholar]
- 75. Sola E, Watson H, Graupera I. et al. Factors related to quality of life in patients with cirrhosis and ascites: relevance of serum sodium concentration and leg edema. J Hepatol 2012;57: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 76. Kalambokis G, Fotopoulos A, Economou M. et al. Effects of a 7-day treatment with midodrine in non-azotemic cirrhotic patients with and without ascites. J Hepatol 2007;46:213–21. [DOI] [PubMed] [Google Scholar]
- 77. Krag A, Moller S, Henriksen JH. et al. Terlipressin improves renal function in patients with cirrhosis and ascites without hepatorenal syndrome. Hepatology 2007;46:1863–71. [DOI] [PubMed] [Google Scholar]
- 78. Fimiani B, Guardia DD, Puoti C. et al. The use of terlipressin in cirrhotic patients with refractory ascites and normal renal function: a multicentric study. Eur J Intern Med 2011;22: 587–90. [DOI] [PubMed] [Google Scholar]
- 79. Wong F, Gines P, Watson H. et al. Effects of a selective vasopressin V2 receptor antagonist, satavaptan, on ascites recurrence after paracentesis in patients with cirrhosis. J Hepatol 2010;53:283–90. [DOI] [PubMed] [Google Scholar]
- 80. Wong F, Watson H, Gerbes A. et al. Satavaptan for the management of ascites in cirrhosis: efficacy and safety across the spectrum of ascites severity. Gut 2012;61: 108–16. [DOI] [PubMed] [Google Scholar]
- 81. Ohki T, Sato K, Yamada T. et al. Efficacy of tolvaptan in patients with refractory ascites in a clinical setting. World J Hepatol 2015;7:1685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Akiyama S, Ikeda K, Sezaki H. et al. Therapeutic effects of short- and intermediate-term tolvaptan administration for refractory ascites in patients with advanced liver cirrhosis. Hepatol Res 2015;45:1062–70. [DOI] [PubMed] [Google Scholar]
- 83. Zhang X, Wang SZ, Zheng JF. et al. Clinical efficacy of tolvaptan for treatment of refractory ascites in liver cirrhosis patients. World J Gastroenterol 2014;20:11400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Torres VE, Chapman AB, Devuyst O. et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012;367:2407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Potosek J, Curry M, Buss M. et al. Integration of palliative care in end-stage liver disease and liver transplantation. J Palliat Med 2014;17:1271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]