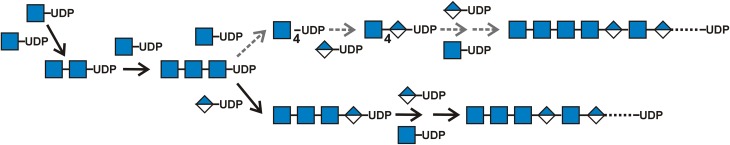
Fig. 13.
The HAS mechanism for the sugar assembly order and linkage during HA chain initiation. HAS always starts the NR-end of a new HA molecule with GlcNAc-UDP (blue squares) and then adds a second GlcNAc-UDP. The enzyme bound to this G–G-UDP product then only accepts another GlcNAc-UDP as the third sugar to make G–G–G-UDP (middle line). After this critical length of three GlcNAc sugars is attained (e.g. to ensure that the nascent chain inserts into the intraprotein pore for successful chain translocation: Tlapak-Simmons et al. 1999; Hubbard et al. 2012), HAS adds either GlcUA (A; blue/white diamonds) to initiate alternating HA disaccharide assembly on a chitin trisaccharide (bottom line, black solid arrows) or HAS adds a fourth GlcNAc to make G–G–G–G-UDP (top line, gray dashed arrows). HAS then adds only GlcUA to initiate alternating HA disaccharide assembly on this chitin tetrasaccharide. Both branched pathways for assembly of G3-UDP or G4-UDP primers allow HAS to start alternating sugar assembly and produce HA chains, the vast majority of which contain a G3 or G4 chitin oligo at their NR-end. In the present studies using SeHAS containing membranes, the chitin trimer was the predominant (97%) NR-end. Initial studies, using HA made by S. pyogenes HAS in live cells, show that a chitin tetramer may be the predominant NR-end. These two results indicate that HAS may be able to create an appropriate length tri- or tetra- GlcNAc primer depending on local membrane conditions (e.g. fluidity changes due to altered temperature or lipid composition) that might influence HAS conformation, pore proximity or the optimal length for entry of a nascent chitin-UDP chain into the intra-HAS pore and effective priming to initiate subsequent HA synthesis.