Summary
Background
To evaluate the safety, etiology and outcomes of patients undergoing bilateral thoracentesis.
Methods
This is a prospective cohort study of 100 consecutive patients who underwent bilateral thoracenteses in an academic medical center from July 2009 through November 2010. Pleural fluid characteristics and etiologies of the effusions were assessed. Mean differences in levels of fluid characteristics between right and left lungs were tested. Associations between fluid characteristics and occurrence of bilateral malignant effusions were evaluated. The rate of pneumothorax and other complications subsequent to bilateral thoracentesis was determined.
Results
Exudates were more common than transudates, and most effusions had multiple etiologies, with 83% having two or more etiologies. Bilateral malignant effusions occurred in 19 patients, were the most common single etiology of exudative effusions, and were associated with higher levels of protein and LDH in the pleural fluid. Among 200 thoracenteses performed with a bilateral procedure, seven resulted in pneumothoraces, three of which required chest tube drainage and four were ex vacuo.
Conclusions
More often than not, there are multiple etiologies that contribute to pleural fluid formation, and of the combinations of etiologies observed congestive heart failure was the most frequent contributor. Exudative effusions are more common than transudates when bilateral effusions are present. Malignancy is a common etiology of exudative effusions. This study suggests that the overall complication rate following bilateral thoracentesis is low and the rate of pneumothorax subsequent to bilateral thoracentesis is comparable to unilateral thoracentesis.
Keywords: Malignancy, Pneumothorax, Thoracic disease, Effusion
Introduction
Pleural effusions result from the accumulation of fluid in the pleural space surrounding the lungs. More than 1.3 million cases occur each year in the United States. The cause of bilateral pleural effusions is generally thought to be due to congestive heart failure (CHF), renal or liver failure, although the only two studies that have objectively evaluated this assumption draw from markedly different populations. In a study conducted over 50 years ago, in cases where patients exhibited normal heart size, only 3.8% of effusions were attributed to congestive heart failure.1 In a separate retrospective study, most cases of bilateral effusions were due to recent coronary artery bypass grafting (CABG) or heart failure.2 That study also showed that, on average, characteristics of the fluid were similar between the left and right sides, including levels of protein, LDH, glucose, and cell counts. Those authors concluded that bilateral fluid analysis need not be performed on both sides unless there is a specific clinical indication.2 There is limited data regarding pleural fluid etiologies or safety of removing fluid from both hemi-thoraces at the same time in patients with bilateral pleural effusions.
The most widely practiced intervention is unilateral thoracentesis, i.e., the removal of fluid from one hemi-thorax with a needle or catheter. Pneumothorax is arguably the most feared complication of thoracentesis and occurs in approximately 6.0% of cases, of which 34% require chest tube placement.1 Iatrogenic risk of pneumothorax subsequent to thoracentesis decreases with the use of ultrasound.3 Whereas unilateral thoracentesis is generally considered to be safe, complications can substantially increase morbidity and cost.
Patients with bilateral pleural effusions may benefit therapeutically from the removal of fluid from both hemi-thoraces. Conventional wisdom dictates that thoracentesis should be performed on one side at a time when bilateral effusions are present, likely from fear of causing bilateral pneumothoraces or other complications. No study has examined the safety of performing concurrent bilateral thoracentesis.
In this observational study we characterized the nature, characteristics, and etiologies of the bilateral effusions. We examined factors associated with the occurrence of bilateral malignant effusions. We also report on rate of complications subsequent to concurrent bilateral thoracentesis in both spontaneously breathing and mechanically ventilated patients.
Materials and methods
This was a prospective cohort study conducted at Yale-New Haven Hospital, a tertiary care medical center. Approval for this study was obtained by the Institutional Review Board. The Thoracic Interventional Program (TIP) is routinely consulted to perform diagnostic and therapeutic thoracenteses for both inpatients and outpatients with pleural effusions. Inpatients were typically on medical floors or in the medical intensive care unit (MICU) rather than surgical floors. When bilateral effusions were present, both hemithoraces were sampled at the same time and fluid was removed until no further drainage occurred. All patients who underwent bilateral thoracentesis between July of 2009 and November 2010 were included after obtaining informed consent. Ultrasound (SonoSite S-ICU, Bothell, WA) was used to identify both effusions and to distinguish the fluid from the surrounding structures, including the lungs, diaphragm and subdiaphragmatic structures. Marks were placed to identify the locations of pleural effusion, with the procedure typically performed posteriorly or along the lateral chest wall. In all awake and cooperative patients, including those who were mechanically ventilated, the procedure was performed with the patient sitting upright and leaning over a table. In those patients who were mechanically ventilated and unable to follow instructions, or in those unable to sit upright, the head of the bed was elevated to 45° and the bilateral thoracenteses were performed from a lateral position.
The patient’s skin was cleaned with chlorhexidine and the procedures were performed in sterile fashion by two operators simultaneously using the Safe-T-Centesis Kit (Cardinal Health, Dublin, OH). Fluid was removed by manual aspiration until it no longer returned. The intent for every thoracentesis was to remove the entire volume of fluid in the pleural cavity. We evacuated the effusion to provide symptomatic relief and to minimize the risk of repeated procedures. Complete removal of the pleural effusion has been endorsed in the literature.4 The fluid volume was measured, and samples were routinely sent for cell count with differential, total protein, lactate dehydrogenase (LDH), glucose, pH, cultures, and cytology.
Light’s criteria were applied to determine whether each effusion was transudative or exudative.2 Etiologies of the pleural effusions were determined from clinical history, laboratory data, volume status and radiology, including echocardiography. Pleural fluid etiologies were categorized according to the schema presented in Table 1. If patients met criteria for more than one etiology they were classified as having multiple etiologies for their pleural effusion. We also compared characteristics of the pleural fluid drawn from left and right sides for disparities in clinical characteristics, i.e., exudative versus transudative.
Table 1.
Definitions for pleural fluid etiology.
| Congestive heart failure: Echocardiogram within 1 month or at the time of procedure which documents:
or
|
CXR = chest X-ray EKG = electrocardiography WBC = white blood cells.
Flow cytometry was performed when lymphoma was a consideration. Effusions were considered to be secondary to malignancy if cytology demonstrated malignant cells or flow cytometry was positive. Cells were considered atypical when the cytopathologist identified abnormal cells but could not definitively characterize them as malignant based on their appearance.
Pre- and post-procedure chest radiographs (CXR) were performed in all patients. The CXRs were read independently by two chest radiologists blinded to the study and patient information. Pneumothorax, worsened edema, worsened infiltrates and improvements in the radiographs were noted when present.
Statistical analysis
Descriptive statistics were ascertained as appropriate and the rate and 95% confidence interval of iatrogenic pneumothoraces subsequent to concurrent bilateral thoracentesis were determined. Paired t-tests of the mean differences in fluid levels of glucose, protein, LDH, and pH between each participant’s right and left effusions were tested against a null value of zero. The unadjusted associations of clinically important covariates and the occurrence of bilateral malignant effusions were determined, and a multivariable logistic model including age and sex was selected with a manual forward selection procedure that has been described previously.5 Goodness of fit was verified by examination of model residuals and with the Hosmer–Lemeshow statistic. We assessed model discrimination by estimating the area under the ROC curve using a C statistic. All statistical tests were two-tailed with P < 0.05 indicating significance. Statistical analyses were performed with SAS statistical software, version 9.2.
Results
Characteristics of participants and their pleural fluid
Of the 100 patients enrolled in this study, 54 were female and 92 were inpatients. A total of 24 patients had the thoracentesis performed either on mechanical ventilation or non-invasive ventilatory support. A descriptive analysis of patient demographics and the contents of their pleural fluid are presented in Table 2. Because there was, on average, a larger volume of effusion aspirated from patients’ right sides, Table 2 presents the level of clinically relevant components from the right-sided effusion only.
Table 2.
Patient demographics (N = 100).
| Characteristic | Mean [SD] or n (%) |
|---|---|
| Age in years, mean [SD] | 70.9 [15.9] |
| Female | 54 (54) |
| Inpatient | 92 (92) |
| Ventilatory support | 25 (25) |
| Mechanical ventilation | 17 (17) |
| Bilevel positive airway pressure (BiPAP) | 7 (7) |
| Pleural fluid results | |
| Bilateral transudates | 35 (35) |
| Bilateral exudates | 53 (53) |
| Bilateral non-matching (exudate & transudate) | 12 (12) |
| Bilateral malignant effusions | 19 (19) |
| Complications recorded by radiologists within 24 h of thoracentesis | |
| Pneumothorax | 7 (7) |
| Edema | 12 (12) |
| Atelectasis | 1 (1) |
| New Infiltrate | 7 (7) |
|
| |
| Analysis of pleural fluid extracted from right side of patients | |
|
| |
| Characteristic | Median {Range} or n (%) |
|
| |
| Fluid volume removed | 875.0 {5–2000} |
| Glucose (mg/dl) | 131.0 {2–282} |
| Lactate dehydrogenase (U/L) | 143.0 {35–2700} |
| Protein (g/dL) | 2.4 {0.6–6.40} |
| pH | 7.46 {7.16–7.63} |
| Granulocytes% | 12.5 {1–98} |
| Lymphocytes% | 25.0 {0–86} |
| Red blood cells | 1460.5 {3–267,000} |
| Microbiology, negative n (%) | 97 (97) |
Missing data: glucose right (n = 2), granulocytes right (n = 1), LDH right (n = 1), pH right (n = 1), microbiology right (n = 1).
Paired t-tests were performed on the levels of protein, LDH, glucose and pH in fluid taken from the right and left sides with no significant difference identified. There was only one empyema and that was in a patient who had an indwelling drainage catheter and no patient had bilateral empyema. Of the 19 patients with cytologically positive bilateral malignant pleural effusions, five had breast adenocarcinoma, five had lung adenocarcinoma and five others were adenocarcinoma from additional primary sites (uterus, pancreas (2), gastric and unknown). The four remaining were pulmonary large cell, lymphoma (2) and T-cell promyelocytic leukemia. An additional five patients had unilateral malignant effusions with cytologic atypia on the contralateral side. All of these were adenocarcinoma (lung (2), colon, gastric and pancreatic). Atypical cells were present bilaterally in five additional patients.
Characterizing the etiology of the bilateral pleural effusions
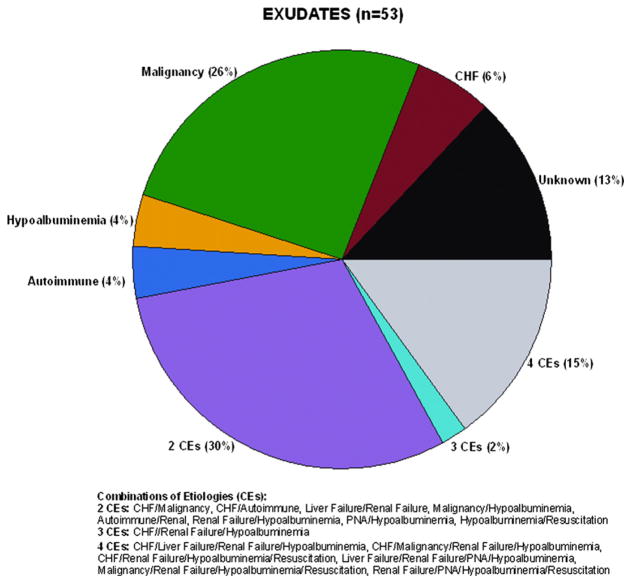
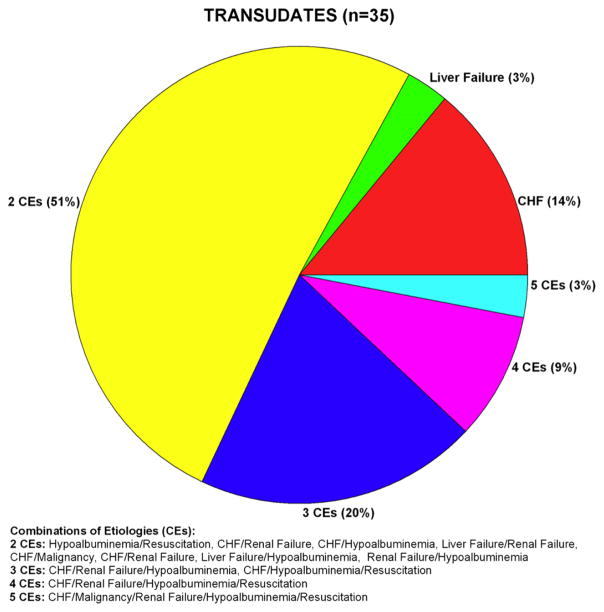
Fifty-three patients had bilateral exudates, 35 had bilateral transudates and there were twelve persons for whom one side was a transudate and the other an exudate. The two pie charts of Figs. 1 and 2 depict, respectively, the proportional breakdown of the attributed etiologies of the exudative and transudative effusions. We only included patients in which both effusions were either transudative or exudative, so the twelve patients who had one side as a transudate and one side as an exudate are not included in Fig. 1. In 13% of effusions no definitive etiology was determined. Forty-seven percent of patients with exudates had more than one etiology for their pleural effusion, of the 15 distinct combinations observed, six included congestive heart failure and ten included renal failure. Of the exudates, malignancy was the most common single etiology, followed by congestive heart failure and a low protein state. Eighty-three percent of patients with bilateral transudates had more than one etiology for their effusion. Of the 12 distinct combinations observed, eight included congestive heart failure and seven included renal failure. Of the transudates in our sample, the most common single etiology was congestive heart failure, followed by liver failure.
Figure 1.
Pie chart of exudative effusion etiologies. Malignancy was the most common single etiology of bilateral exudates. The majority of patients had multiple etiologies for their effusions.
Figure 2.
Pie chart of transudative effusion etiologies. Heart failure was the most common single etiology of the bilateral transudates. The majority of patients had multiple etiologies for their effusions.
Modeling the occurrence of bilateral malignant effusion
With 19 patients exhibiting bilateral malignant effusions, it was the most common cause of exudative effusion. We examined the unadjusted associations between characteristics of the effusions and occurrence of bilateral malignant effusion. Table 3 provides the bivariate associations between patient characteristics and the occurrence of bilateral malignant effusion calculated from logistic regression. As was done for Table 2, we report associations with the fluid characteristics of bilateral malignant effusion from the right side only. Among the demographic characteristics, age alone showed a marginal association. We observed that higher levels of protein and LDH were significantly associated with occurrence of bilateral malignant effusions. Correspondingly, lower levels of glucose and pH demonstrated significant bivariate associations. A positive diagnosis of malignancy was precluded from use in the models because all but one case of bilateral malignant effusion were characterized by previous diagnoses of malignancy. We subsequently fit a multivariable model that consisted of age, sex, protein and glucose. Among these four explanatory variables, only protein and glucose exhibited marginal associations with P-values of 0.05 and 0.06, respectively. With a C statistic of 0.82 and no rejection of Hosmer–Lemeshow goodness of fit, this multivariable model showed good ability to predict occurrence of bilateral malignant effusions.
Table 3.
Bivariate associations between patient characteristics and occurrence of bilateral malignant effusion (N = 100).
| Mean or prevalence | Coefficient | P-value | |
|---|---|---|---|
| Demographics | |||
| Age in years, mean ± SD | 70.9 (15.9) | −0.03 | 0.04 |
| Female gender | 0.54 | −0.59 | 0.25 |
| Serum values | |||
| Protein | 5.8 (1.0) | 0.14 | 0.61 |
| Lactate dehydrogenase | 303.6 (136) | −0.001 | 0.61 |
| Analysis of pleural fluid extracted from right side of patients | |||
| Glucose | 133.1 (47) | −0.02 | <0.001 |
| Lactate dehydrogenase | 267.2 (388.2) | 0.002 | 0.003 |
| Protein | 2.49 (1.2) | 0.83 | <0.001 |
| pH | 7.44 (0.1) | −6.85 | 0.02 |
| Granulocytes% | 22.9 (23.1) | −0.03 | 0.02 |
| Lymphocytes% | 31.7 (24.9) | −0.003 | 0.80 |
| Red blood cells | 13,775.3 (36739.7) | −0.0001 | 0.01 |
| Microbiology, negative | 0.02 | 1.5 | 0.32 |
Safety of concurrent bilateral thoracentesis
Of the 200 concurrent bilateral procedures performed, seven resulted in pneumothoraces, representing a rate (95% CI) of 3.6% (1.7, 7.7). In our sample, the number of pneumothoraces requiring chest tube placement was three, representing 1.5% of all procedures. In all cases, an experienced operator was available to handle complications and none of the patients developed persistent bronchopleural fistula or died. All pneumothoraces occurred on the right side and were characterized by removal of larger volumes of fluid. The volume of fluid removed was more than 1.5 L in each of our cases of pneumothorax. The four other pneumothoraces not requiring chest tube placement represented “ex vacuo” spaces in which the lung did not re-expand. The causes of ex vacuo pneumothoraces included one patient with a perforated atrium following a pacemaker placement, one patient with an inferior vena cava stenosis and chronic effusions following treatment for lymphoma, and one patient with bilateral malignant effusions.
Discussion
Pleural effusions are a common clinical problem in medical practice and occur in patients with heart, liver, and renal failure as well as malignancy. Prior literature suggests that effusions can be bilateral in anywhere from 15 to 55% of patients.2 Prior to this study, there has been little published information regarding the etiologies or safety of bilateral thoracentesis.
Etiologies of bilateral pleural effusions
In this study most of the bilateral effusions, whether exudative or transudative, were attributed to multiple etiologies; 83% of the transudates and 47% of the exudates respectively. In the current study, 19 patients (19%) had malignancy as a cause of bilateral effusion. In two previously described studies, malignancy was found in 35 patients (45%) among the first study’s general population,1 but no malignancy was identified in the second study.2 Only one patient with bilateral malignant effusions in our study had no prior history of malignancy. Of the patients with bilateral malignant effusions, one patient had bilateral transudative effusions, one patient had a transudate and an exudate and all others were bilateral exudative effusions. This is consistent with the literature reporting that the majority of malignant effusions are exudates. Patients with bilateral pleural effusions and a history of malignancy should raise suspicion for malignant involvement of both hemithoraces.
Our analysis of fluid drawn simultaneously from both lungs showed no statistical difference between sides in traditionally measured components used to determine whether the fluid is exudative versus transudative. This finding was also reported by Kalomenidis et al.2 In contrast with that cardio-centric sample, our bilateral samples were drawn from a more diverse medical population and may therefore suggest greater generalizability of the observed similarity in makeup of fluid from both effusions. In rare circumstances, the etiology of the pleural effusion was different between sides. For example, unilateral pneumonia requiring systemic fluid resuscitation led to different pleural fluid characteristics in which only the side with pneumonia was exudative. In another case, one side was chylous. In another, cholecystectomy led to a right-sided inflammatory effusion, while the left side was a transudate. Finally, one patient had a unilateral malignant effusion and developed bilateral effusions after resuscitation for bacteremia; in this case, the contralateral effusion was not malignant. Sometimes, the reason for differences between sides was not clearly determined on clinical grounds.
Our results support findings from other studies regarding which tests to include in pleural fluid analysis. Routine pleural fluid cultures may not be needed in the presence of bilateral effusions without a clear clinical picture of pneumonia. In our study, only four patients had pneumonia and only one effusion was culture-positive, the latter effusion caused by an indwelling tube.
Safety of concurrent bilateral thoracentesis
There is disagreement in the literature regarding the adverse outcomes associated with large volume removal of pleural fluid. The two main adverse outcomes are pneumothorax and re-expansion pulmonary edema. Many factors likely contribute to these adverse outcomes including etiology of the effusion, how long the effusion has been present and technique of removal. A study by Josephson et al. demonstrated that pneumothorax was more common in patients that had greater than 1.8 L removed.6 In that study there were 735 thoracenteses performed in 471 patients suggesting recurring development of pleural fluid in their population so that repeated thoracentesis and not the volume removed could have been the true risk factor for pneumothorax. Several other studies have not found an association with volume removal and pneumothorax or re-expansion pulmonary edema.4,7 The most recent British Thoracic Society guidelines acknowledge that the amount of fluid that can be safely removed is debatable.8
Our observational sample of 100 patients suggests that ultrasound-guided bilateral thoracentesis demonstrates safety comparable with that of unilateral thoracentesis. Our rate of pneumothorax was 3.6%, which is lower than the rate reported by Gordon et al.3 for unilateral thoracentesis, i.e., 6.0% (CI: 4.6, 7.8). They also reported that 2% of all procedures required chest tube placement, similar to our 1.5% rate of chest tube placement. Four patients did not require chest tube placement because their pneumothorax was considered ex vacuo. This is in accordance with the British Thoracic Society guidelines.8 In addition a study by Boland et al. demonstrated improvement in patient symptoms with fluid removal despite occurrence of an ex vacuo pneumothorax.9 All of our pneumothoraces occurred when greater than 1.5 L of fluid was removed. This finding is similar to a prior study in which the pneumothorax rate was higher for effusions that were 1.8–2.2 L (three-fold increase) or more than 2.2 L (six-fold increase).6 It should be noted, however, that in our study the great majority of patients with more than 1.5 L of pleural fluid removed did not develop a pneumothorax. Twenty-four of our patients, representing 48 thoracenteses, underwent their procedure while on positive pressure ventilation without an increased rate of adverse outcomes and only one had a pneumothorax requiring intervention. Therefore, contrary to conventional wisdom, we propose that thoracenteses can be performed safely in both hemithoraces at the same time, even in mechanically ventilated patients. Until further data is available it may be prudent to remove less than 1.5 L from each hemithorax when performing a bilateral thoracentesis.
There are several plausible benefits from performing concurrent bilateral thoracentesis. They include reduction in the overall time spent by physician and medical staff in positioning the patient, as well as faster relief of symptoms. The concurrent bilateral procedure also reduces patient exposure to radiation as it eliminates replication of radiographs in those cases where the physician orders a CXR following each unilateral procedure. Given that prior studies have demonstrated improvement in FEV1, FVC and arterial oxygenation with removal of fluid volumes ranging from 600 to 2700 ml, the same effects were likely occurring in our sample.10 These changes in lung function may lead to improved symptoms and patient satisfaction, as well as earlier diagnosis and hospital discharge.
There are notable limitations to this study. Because the primary outcomes of this study were determining etiologies and safety, patient symptoms were not quantitatively measured before and after the procedure. The magnitude of therapeutic benefit was therefore not directly assessed. Future studies should document improvement in symptoms with bilateral thoracentesis. This study was conducted in a medical population whereas a post-surgical population may have other etiologies for their bilateral effusions. Another limitation is that we did not systematically capture the clinician’s reason for requesting a bilateral thoracentesis although for many of the cases it was due to refractory effusions that were not responding to diuretic therapy or hemodialysis, unexplained fever or for symptomatic relief. Although in our study two operators performed the thoracentesis at the same time, bilateral thoracentesis could be performed with ultrasound guidance in an immediate sequential manner by one operator without performing a chest radiograph between the procedures. This approach would be practical in a community setting where an experienced clinician using ultrasound could do serial thoracentesis without taking excess time to reposition the patient or to wait for separate radiographs to be performed between procedures.
Conclusions
In most patients, multiple etiologies contributed to the information of bilateral pleural effusions. In the absence of a unilateral pneumonia, unilateral subdiaphragmatic surgical procedures, or obvious reasons for dissimilar results, most bilateral pleural effusions are anticipated to have similar fluid characteristics and etiologies. In this observational prospective cohort study, concurrent bilateral thoracentesis demonstrates a rate of iatrogenic pneumothoraces comparable to that of unilateral thoracentesis. Concurrent bilateral thoracentesis can be safely performed with the guidance of ultrasound and the manual evacuation of fluid with at least one experienced operator present.
Acknowledgments
The authors thank Kelsey Johnson, PA, for her diligent care of these patients and her participation in performing many of these procedures. No financial support for this study was received. K. Araujo and T.E. Murphy were supported by the National Institute on Aging through the Claude D. Pepper Older Americans Independence Center (P30AG21342) at Yale University.
Footnotes
Author contribution
Conception and Design: JP, MP, CA. Analysis and Interpretation: JP, CA, TM, KA, IO, AR, MP. Drafting of Manuscript and important intellectual content: JP, CA, TM, MP.
Conflicts of interest
None.
References
- 1.Blackman NS, Rabin CB. Bilateral pleural effusion; its significance in association with a heart of normal size. J Mt Sinai-Hosp NY. 1957;24(1):45–53. [PubMed] [Google Scholar]
- 2.Kalomenidis I, Rodriguez M, Barnette R, et al. Patient with bilateral pleural effusion: are the findings the same in each fluid? Chest. 2003;124(1):167–76. doi: 10.1378/chest.124.1.167. [DOI] [PubMed] [Google Scholar]
- 3.Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332–9. doi: 10.1001/archinternmed.2009.548. [DOI] [PubMed] [Google Scholar]
- 4.Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656–61. doi: 10.1016/j.athoracsur.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 5.Van Ness PH, Murphy TE, Araujo KL, Pisani MA, Allore HG. The use of missingness screens in clinical epidemiologic research has implications for regression modeling. J Clin Epidemiol. 2007;60(12):1239–45. doi: 10.1016/j.jclinepi.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42–7. doi: 10.1080/02841850802590460. [DOI] [PubMed] [Google Scholar]
- 7.Colt HG, Brewer N, Barbur E. Evaluation of patient-related and procedure-related factors contributing to pneumothorax following thoracentesis. Chest. 1999;116(1):134–8. doi: 10.1378/chest.116.1.134. [DOI] [PubMed] [Google Scholar]
- 8.Havelock T, Teoh R, Laws D, Gleeson F. Pleural procedures and thoracic ultrasound: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii61–76. doi: 10.1136/thx.2010.137026. [DOI] [PubMed] [Google Scholar]
- 9.Boland GW, Gazelle GS, Girard MJ, Mueller PR. Asymptomatic hydropneumothorax after therapeutic thoracentesis for malignant pleural effusions. AJR Am J Roentgenol. 1998;170(4):943–6. doi: 10.2214/ajr.170.4.9530040. [DOI] [PubMed] [Google Scholar]
- 10.Wang JS, Tseng CH. Changes in pulmonary mechanics and gas exchange after thoracentesis on patients with inversion of a hemidiaphragm secondary to large pleural effusion. Chest. 1995;107(6):1610–4. doi: 10.1378/chest.107.6.1610. [DOI] [PubMed] [Google Scholar]