Abstract
Prior studies have concluded that schizophrenia patients are not anhedonic because they do not report reduced experience of positive emotion to pleasant stimuli. The current study challenged this view by applying quantitative methods validated in the Evaluative Space Model of emotional experience to test the hypothesis that schizophrenia patients evidence a reduction in the normative “positivity offset” (i.e., the tendency to experience higher levels of positive than negative emotional output when stimulus input is absent or weak). Participants included 76 schizophrenia patients and 60 healthy controls who completed an emotional experience task that required reporting the level of positive emotion, negative emotion, and arousal to photographs. Results indicated that although schizophrenia patients evidenced intact capacity to experience positive emotion at high levels of stimulus input, they displayed a diminished positivity offset. Reductions in the positivity offset may underlie volitional disturbance, limiting approach behaviors toward novel stimuli in neutral environments.
Keywords: Anhedonia, Emotion, Affective Ambivalence, Psychosis
Introduction
Anhedonia, traditionally defined as a diminished capacity to experience pleasure, has been considered a core component of schizophrenia since the earliest conceptualizations of the disorder (Kraepelin, 1919; Bleuler, 1911; Rado, 1953). However, modern empirical evidence provides mixed support for the presence of anhedonia in schizophrenia (Kring & Moran, 2008). On the one hand is data resulting from clinical interviews indicating that the majority of schizophrenia patients meet criteria for mild or greater severity of anhedonia (Strauss, 2014). However, seemingly contradictory evidence has emerged from laboratory-based and experience sampling studies, which indicate that schizophrenia patients report levels of positive emotion (Cohen & Minor, 2010) and arousal (Llerena, Strauss, Cohen, 2012) that are nearly identical to healthy controls when exposed to pleasant stimuli or real-world activities (Gard et al., 2007; Oorschot et al., 2013). This apparent paradox can be resolved via careful inspection of probes and anchors used during clinical interviews, which tend to focus on the frequency with which patients engage in pleasurable activity (i.e., behavior), rather than intensity of positive emotion patients experience when actually engaged in activities (i.e., capacity) (Strauss & Gold, 2012). Ecological Momentary Assessment studies, which examine emotional experience in the context of real-world activity, also indicate that anhedonia may be better understood as a reduction in pleasurable behavior than a diminished capacity because people with schizophrenia report increases in positive emotion that are intense as controls when engaged in activities, despite not engaging in activities as frequently (Gard et al., 2007, 2015; Oorschot et al., 2011).
These findings lead to an important question: why do apparently normal hedonic experiences not translate into motivated behaviors aimed at obtaining rewards? Several models of reward processing have been proposed to answer this question (Gold et al., 2008; Cohen et al., 2011; Ursu et al., 2011; Barch & Dowd, 2010; Strauss, Waltz, Gold, 2013; Kring & Ellis, 2013; Kring & Barch, 2014). These models all rely on the assumption that the experience of positive emotion is normal in schizophrenia, and propose that impairments in several aspects of reward processing (e.g., value representation, effort-cost computation, reinforcement learning) other than initial hedonic response lead to reductions in goal-directed activity. Although these models have received considerable empirical support (Kring & Barch, 2014), it is possible that conclusions regarding affective normality in schizophrenia are premature. Perhaps abnormalities in emotional experience do contribute to motivational impairments in schizophrenia (i.e., in addition to abnormalities in other aspects of reward processing), but the theoretical frameworks and methods used to evaluate hedonic response in past studies were not sophisticated enough to detect the abnormalities that exist in this population.
In the current study, we tested this possibility by adopting a conceptual framework that has been well validated in the field of affective science, but not applied to schizophrenia, the “Evaluative Space Model” (Cacioppo & Berntson, 1994). In brief, the Evaluative Space Model proposes that separate motivational systems govern self-reported positive and negative evaluative responses. Each motivational system is characterized by an activation function that translates emotional responses into motivated behavior. In this framework, activation function refers to the relationship between the affective input into the system and the output from that system. For example, imagine that the positive and negative systems are two separate audio speakers that have different volume dials (i.e., inputs) capable of controlling decibel levels from the speaker (i.e., output). It is possible for the two speakers to be calibrated perfectly such that a one unit increase in input results in the same degree of change in output in both speakers; in such a case, the two systems have symmetrically calibrated activation functions. However, symmetrical calibration is not how the affective system functions in healthy individuals. Years of evolution have produced positive and negative affective systems that are differentially calibrated to respond in specific environmental contexts where they are most adaptive. Since unpleasant stimuli have stronger implications for survival than equally intense pleasant stimuli (e.g., it is more beneficial to respond to threats from a foe than pursue an attractive mate), the affective system is calibrated to respond with incrementally greater levels of negative than positive emotion as the level of emotional input increases (Cacioppo & Berntson, 1994; Larsen, McGraw, Cacioppo, 2001; Ito & Cacioppo, 2005; Norris et al., 2010). Put differently, in relation to the speaker example, a one-unit increase in the volume dial produces a comparatively louder decibel output on the negative than positive speaker. The Evaluative Space Model terms this tendency the “negativity bias”. In contrast, the positive system is characterized by what has been termed the “positivity offset”, which reflects the tendency of the positive system to respond more strongly than the negative system in the absence of emotional input or when levels of input are weak (Cacioppo & Berntson, 1994; Larsen, McGraw, Cacioppo, 2001; Ito & Cacioppo, 2005; Norris et al., 2010). Returning to the speaker example, the positivity speaker either continues to emit a low level output (e.g., hum) when it is turned off, or it produces a higher output than the negativity speaker when input is low. The result of this imbalance in calibration is that healthy individuals tend to experience a greater balance of positive than negative emotion in most situations, which tend to be neutral and characterized by little or no affective input. The positivity offset is adaptive because it promotes exploratory behavior and the approach of novel stimuli in neutral environments that can allow for acquisition of new rewards and resources. Thus, the evaluative space model proposes that asymmetries in the activation functions of positive and negative emotion have differentially evolved to produce motivated behaviors that are contextually adaptive.
Support for these tenets of the Evaluative Space Model comes from an extensive literature. First, laboratory-based studies examining individual differences in self-report to affective stimuli support the existence of both constructs, as well as their utility. In these studies, the positivity offset and negativity bias have been operationalized quantitatively as parameters within regression equations (Ito & Cacioppo, 2005). Strength of positive and negative self-reports (affective output) is predicted from arousal ratings, which denote the level of evaluative input into the affective system. The positivity offset is defined as the intercept value for positivity relative to negativity (i.e., the output at zero input), with higher intercept values for positive than negative emotion indicating the presence of the normative “positivity offset”. The negativity bias is defined as the slope value for negative compared to positive emotion, with greater rate of change in output of negative emotion per unit of input than positive emotion (i.e., steeper slope for negative than positive emotion). Positivity offset and negativity bias scores calculated using these regression parameters have demonstrated good temporal stability, internal consistency, and discriminant validity (Ito & Cacioppo, 2005). The positivity offset and negativity bias have been found using a variety of modalities (e.g., auditory, visual) and stimulus types (e.g., photographs, words) (Norris, Larsen, Crawford, & Cacioppo, 2011; Larsen, Norris, McGraw, Hawkley, & Cacioppo, 2009), and individual differences in the positivity offset and negativity bias predict neural response during neuroimaging tasks, learning of relationships between affect and spatial location, judgments on an impression formation task, and serotonin receptor gene expression (Ito & Cacioppo, 2005; Norris et al., 2010; Ashare et al., 2013). Second, support for the negativity bias and positivity offset comes from a variety of psychological studies using other methods. For example, the negativity bias is supported by evidence indicating that at comparable levels of high stimulus arousal, there is greater response for unpleasant than pleasant stimuli in psychophysiological studies measuring autonomic nervous system functioning, cognitive studies measuring attention-emotion interactions, and neuroimaging or electrophysiological studies examining neural response (Cacioppo et al., 2000; Carretie et al., 2001; Cunnigham et al., 2008; Gollan et al., 2015; Ito et al., 1998; Neta, Norris, Whalen, 2009; Norris et al., 2010; Smith et al., 2006; Taylor, 1991). Decision-making studies on framing effects also indicate that monetary losses “loom larger” than gains when large outcomes are at stake, such that potential losses affect behavior moreso than potential rewards (Kahneman & Tversky, 1984). The positivity offset is also supported by evidence indicating the presence of the mere exposure effect (i.e., when a stimulus is repeated frequently, repetition increases positive emotion toward the repeated stimulus) (Zajonc, 1968), that positive words are used more frequently in the English language than negative words (Boucher & Osgood, 1969), the person positivity effect (i.e., mild positive emotion experienced to unknown others) (Sears, 1983), and framing studies indicating that gains loom larger than losses under conditions where smaller outcomes are at stake (Harinck et al., 2007). Thus, multiple levels of evidence support the existence of a positivity offset at low levels of affective input and a negativity bias at high levels of affective input. The failure to flexibly calibrate these activation functions across different contexts is thought to contribute to different forms of psychopathology (e.g., depression; Gollan et al., 2015, 2016); however, the evaluative space model has not yet been applied to study schizophrenia.
Even though traditional analyses indicate that the mean level of positive emotion to pleasant stimuli is comparable between schizophrenia patients and controls (Cohen & Minor, 2010), it may still be possible for schizophrenia patients to have affective abnormalities from the perspective of the Evaluative Space Model. For example, consider the possibility that schizophrenia patients have a lower intercept value for positive emotion than controls, signifying weaker output responses to pleasant stimuli when affective input is low or absent. If patients also have steeper slope values for positivity than controls, this would explain why groups do not exhibit mean differences in positive emotion to an aggregate set of pleasant stimuli when traditional univariate analyses are applied. Such evidence would also explain the inherently paradoxical problem of how schizophrenia patients can have an intact capacity for pleasure, yet still not frequently seek out rewarding activities (i.e., the level of positive emotion is not high enough to facilitate exploratory and approach behavior in environments with low affective input, even though hedonic output becomes normal when affective input is high). Alternatively, schizophrenia may be characterized by an abnormality in the ratio of positive to negative emotion when affective input is low or absent (i.e., a failure to display the prototypical finding of higher intercept scores for positive than negative emotion). If the ratio of positive to negative emotion is too low, or even inverted (i.e., higher intercept for negative than positive emotion), patients might be less likely to engage in approach behaviors. A certain “purity” of positive emotional experience (i.e., untainted by concurrent negative emotion) may be required to generate the motivational force needed to seek out potential rewards. Finally, it may be that schizophrenia is primarily characterized by an abnormality in negative, rather than positive emotional experience (Cohen et al., 2011). In the Evaluative Space Model, this could be indicated by higher “negativity bias” scores in patients than controls, indicating that people with schizophrenia experience a steeper increase in negative emotion as the level of affective input increases (i.e., higher slope values for negative emotion in schizophrenia patients). A heightened negativity bias could be maladaptive, leading patients to experience more negative emotion than what is called for in response to most stimuli. Thus, there are numerous means by which hedonic abnormalities could manifest in schizophrenia when evaluated through the lens of the Evaluative Space Model, even if traditional analyses of the level of positive emotion to pleasant stimuli indicate no group difference between patients and controls.
Another critical component of the Evaluative Space Model is the concept of emotional “co-activation”. Numerous laboratory and experience sampling studies have demonstrated that positive and negative emotions are not diametric opposites (Norman et al., 2011). Rather, positivity and negativity are separable and partially distinct components of the affect system that can be experienced simultaneously, making it possible to experience co-activations of positive and negative emotion (e.g., feeling elated to graduate from college, but simultaneously afraid of the prospect of what the future holds). The combined level of positive and negative emotion experienced at any time point has an important influence on every-day activities, determining whether individuals engage in adaptive approach/avoidance behaviors or remain inactive (Larsen, McGraw, Cacioppo, 2001). Bleuler (1911) proposed that affective ambivalence, a concept similar to co-activation, may be core to schizophrenia and a key ingredient in avolition. Consistent with this notion, there is some evidence for affective incongruence in schizophrenia (i.e., people with schizophrenia experience greater levels of positive emotion to unpleasant stimuli and greater levels of negative emotion to pleasant stimuli than controls) (Tremeau et al., 2010; Sanchez et al., 2014; Strauss & Herbener, 2011); however, prior studies have yet to quantitatively demonstrate that individuals with schizophrenia experience greater “co-activation” than healthy controls (i.e., concurrent increases in both positive and negative emotion to the same stimulus). In the current study, we evaluated whether individuals with schizophrenia have increased co-activation using vector-based equations that have been validated in prior investigations of the Evaluative Space Model (Norman et al., 2011). These analyses plot separate unipolar ratings of positive and negative emotion for pleasant, unpleasant, and neutral stimuli as points within a two-dimensional Cartesian coordinate system. Two orthogonal vector parameters were calculated to examine co-activation: vector angle and vector magnitude. Vector angle was determined by evaluating the deviation (up to + or − 45 degrees) of each participant’s rating of positivity and negativity of a stimulus from the reciprocal diagonal. Vector angle scores of 0 reflect perfect co-activation for a stimulus (i.e., balanced positivity and negativity), whereas positive vector angle values reflect a predominance of positivity judgments and negative angles reflect a predominance of negativity judgments. The second measure, vector magnitude, was calculated as the hypotenuse of the positivity and negativity ratings placed in two-dimensional evaluative space. Values reflect the overall evaluative distance from zero, with higher scores indicating greater co-activation of positive and negative emotion to a stimulus. When considered in tandem, vector angle and magnitude scores can be used to determine whether individuals with schizophrenia experience greater co-activation than controls. Co-activation would be indicated by higher vector magnitude scores in schizophrenia patients than controls, as well as mean vector angle scores that fall around zero in the schizophrenia group.
The current study provides the first application of the evaluative space model to study emotional experience in schizophrenia. The following hypotheses were made: 1) Schizophrenia patients would fail to evidence the normative “positivity offset”, as indicated by lower intercept scores for positivity ratings and a lower positive/negative ratio intercept score than controls; 2) Schizophrenia patients would display a greater “negativity bias” than controls, as indicated by steeper slope values for negativity ratings than controls; 3) Schizophrenia patients would evidence increased co-activation of positive and negative emotion compared to controls, as indicated by vector angle scores close to zero and greater vector magnitude scores than controls; 4) Positivity offset, negativity bias, and co-activation scores would significantly predict self-reported and clinically rated anhedonia in schizophrenia patients.
Method
Participants
Participants included 76 individuals meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR: American Psychiatric Association, 2000) criteria for schizophrenia or schizoaffective disorder and 60 healthy controls. Patients were recruited from the outpatient research clinics at the Maryland Psychiatric Research Center and community mental health clinics in the Baltimore metropolitan area. All patients were evaluated during periods of clinical stability as determined by a minimum of 4-weeks of consistent medication dose and type. Consensus diagnosis was established via a best-estimate approach based on psychiatric history and subsequently confirmed using the Structured Clinical Interview for DSM-IV (SCID: First et al., 1997).
Control subjects were recruited through random-digit dialing, posted advertisements, or word of mouth among enrolled participants. All controls underwent a screening interview, including the SCID-I and SCID-II (Pfol, Blum, Zimmerman, 1997) and did not meet current criteria for any current Axis I disorder or an Axis II schizophrenia-spectrum disorder. Controls also had no family history of psychosis. Additional exclusion criteria for all participants were: lifetime neurological conditions (e.g., traumatic brain injury, epilepsy), DSM-IV criteria for substance dependence within the past 6 months, and recent drug use as determined by urine toxicology screening.
Participants with schizophrenia and controls did not differ on age, sex, or ethnicity; however, patients had significantly lower personal education than controls (see Table 1). Data on income or socioeconomic status was not collected.
Table 1.
Participant Demographics
| Schizophrenia (n = 76) | Control (n =60) | Test Statistic, p-value | |
|---|---|---|---|
| Age | 43.2 (11.5) | 40.9 (10.6) | F = 1.4, p = 0.25 |
| Education | 13.0 (2.0) | 15.6 (1.9) | F = 56.7, p < 0.001 |
| % Male | 67.1% | 65.0% | 0.64, p = 0.73 |
| Race % | 4.47, p = 0.61 | ||
| Caucasian | 69.7% | 61.7% | |
| African-American | 22.4% | 31.7% | |
| American-Indian | 2.6% | 0% | |
| Asian-American | 1.3% | 1.7% | |
| Mixed-Race | 1.3% | 0% | |
| Other | 2.6% | 5.0% |
Procedures
Data were pooled across 3 studies that used similar protocols and tasks1: Experiment 1- Schizophrenia: n = 40; Control: n = 34; Experiment 2- Schizophrenia: n = 16; Control: n = 22; Experiment 3- Schizophrenia: n = 20; Control: n = 4. There was no overlap in participants across the 3 studies. Each study used different numbers and subsets of stimuli from the International Affective Picture System (IAPS: 25) library (see below). Participants first completed a clinical interview administered by a clinical psychologist (GPS) trained to reliability standards on gold standard videos (reliability > 0.80), after which participants with schizophrenia were rated on the Brief Psychiatric Rating Scale (BPRS: Overall & Gorham, 1962), Brief Negative Symptom Scale (BNSS: Kirkpatrick et al., 2011), and Level of Functioning Scale (LOF: Hawk, Carpenter, Strauss, 1975). The Chapman Physical and Revised Social Anhedonia questionnaires (Chapman, Chapman, Raulin, 1976; Eckblad et al., 1982) were used to evaluate trait self-reported anhedonia. The emotional experience task was then administered.
Emotional Experience Task
A common Emotional Experience paradigm was used across all 3 experiments. Participants were exposed to pleasant, unpleasant, and neutral images from the IAPS (Lang, Bradley, Cuthbert, 2008) and asked to make three judgments on each trial: How positive does the picture make you feel?; How negative does the picture make you feel?; How calm/excited does the picture make you feel (i.e., subjective arousal)? The trial first started with a fixation cross, which was followed by a prompt denoting the type of report (e.g., how positive?) that remained on screen with the picture until the participant made a rating. Unlimited time was given for each rating. The self-assessment manikin (SAM) was presented below the image for each rating. In all experiments, SAM ratings were made on a 1 (not at all) to 9 (extremely) scale for ratings of positive and negative emotion and 1 (extremely calm) to 9 (extremely excited) scale for arousal. Participants made manual responses using a keyboard for all 3 self-reports. The order of the 3 self-reports was kept constant on every trial to reduce cognitive demand (i.e., how positive, how negative, arousal).
The number of stimuli used in practice and experimental phases differed across experiments. In Experiment 1, participants rated a total of 3 practice stimuli (1 pleasant, 1 unpleasant, 1 neutral) and 90 experimental stimuli (30 pleasant, 30 unpleasant, 30 neutral). In Experiment 2, there were a total of 3 practice stimuli (1 pleasant, 1 unpleasant, 1 neutral) and 24 experimental stimuli (8 pleasant, 8 unpleasant, 8 neutral). In Experiment 3, there were 3 practice and 120 experimental stimuli (40 pleasant, 40 unpleasant, 40 neutral). Practice stimuli were not included in the experimental blocks.
In each of the 3 experiments, pleasant, unpleasant, and neutral stimuli differed in normative IAPS valence (unpleasant < neutral < pleasant) and arousal (neutral < pleasant, unpleasant). Pleasant and unpleasant stimuli did not significantly differ in normative arousal. In all 3 experiments, images depicted social and nonsocial content. Unpleasant images consisted of threat, injury, disgust, and phobic scenes. Pleasant images included beautiful landscapes, food, romantic scenes, animals, adventure, and erotica. Neutral scenes consisted of common objects, expressionless people, and nature.
Positivity Offset and Negativity Bias Calculations
Our method for calculating the positivity offset and negativity bias was based on Ito and Cacioppo (2005). These variables were conceptualized in terms of regression parameters. Positivity offset was calculated as the intercept for positivity (i.e., output at zero input) and the negativity bias calculated as the slope for negativity (i.e., rate of change in output for unit of input). Two separate regression analyses were calculated for each group. For the positivity function, mean arousal for pleasant and neutral stimuli was used as the predictor, and mean positivity ratings for pleasant and neutral stimuli served as the dependent variable. For the negativity function, mean arousal for unpleasant and neutral stimuli served as the predictor, and mean negativity rating for unpleasant and neutral stimuli served as the dependent variable. As done in Ito and Caccioppo (2005), arousal was selected to index the level of affective input of the stimulus. Arousal ratings made by each participant were used instead of normative IAPS arousal ratings because normative ratings are known to differ between middle-aged community participants (such as those in the current study) and undergraduate students (such as those that the IAPS was normed on).
To determine whether individual differences in the positivity offset and negativity bias predicted anhedonia, two separate regression analyses were calculated for each subject using data from individual trials. Similar to the group level analyses, mean arousal for pleasant and neutral stimuli was used as the predictor for the positivity function, and mean positivity ratings for pleasant and neutral stimuli was the dependent variable. Mean arousal for unpleasant and neutral stimuli was the predictor for the negativity function, and mean negativity rating for unpleasant and neutral stimuli was the dependent variable. Individual differences in the positivity offset were examined using a difference score of positive intercept – negative intercept, whereas negativity bias was indexed as each participant’s slope for the negativity function.
Higher positivity offset scores therefore reflect greater levels of positive than negative emotional output at low levels of affective input. Negativity bias scores reflect the magnitude of increase in negative emotion output per unit of increase in affective input.
Vector Calculations used to Examine Co-Activation
Within the Evaluative Space Model, individual positivity and negativity ratings can be characterized by 2 orthogonal vector parameters: angle and magnitude. Equations were based on (Norman et al., 2011). Vector magnitude indicates the overall evaluative distance from zero, calculated as the hypotenuse of the positivity and negativity ratings placed in evaluative space using a two-dimensional Cartesian coordinate system. Vector magnitude was used here to index degree of co-activation of positive and negative emotion to a stimulus, with higher scores indicating greater co-activation.
Vector angle represents deviation (up to + or − 45 degrees) from the reciprocal diagonal, which was calculated as the interior angles of the junction between the 0 degree line bisecting the planes and the ordinate. Positive vector angle values reflect a predominance of positivity judgments, whereas a negative angle reflects a predominance of negativity judgments. A score of 0 reflects co-activation or balanced positivity and negativity.
Using vector angle and vector magnitude calculations, co-activation would be indicated by higher vector magnitude scores and vector angle scores for all stimuli that fall around zero.
Results
Traditional Valence and Arousal Analyses
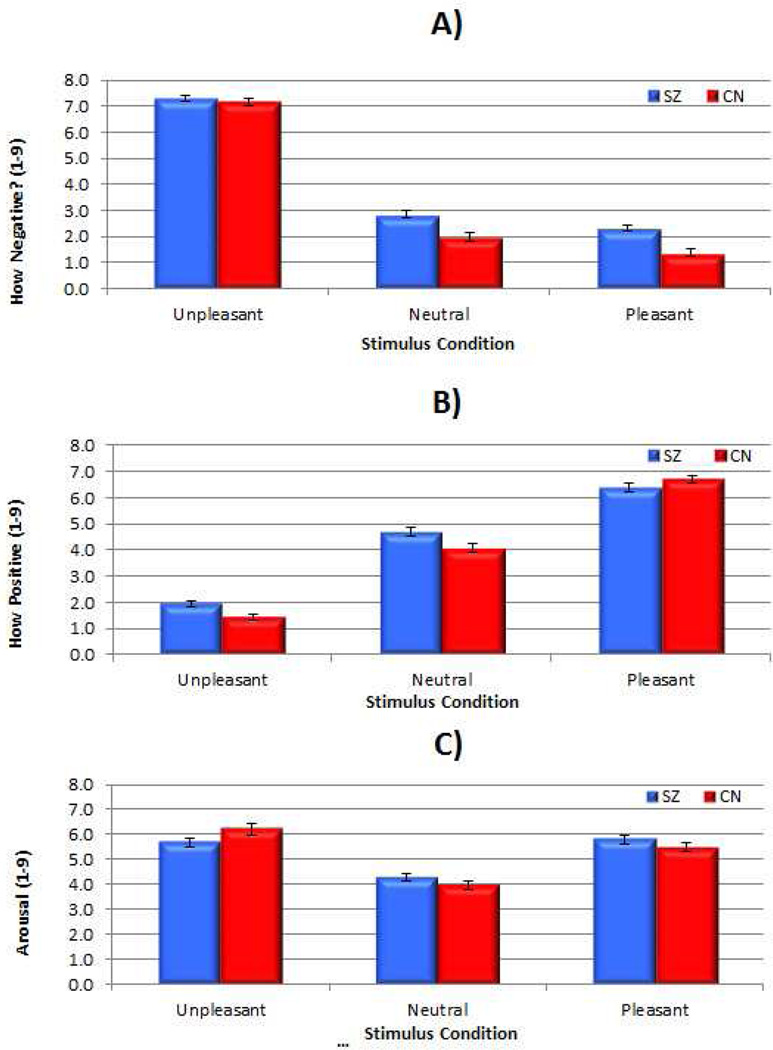
To determine comparability of the current results to past studies, we first conducted standard analyses of self-reports of how positive, how negative, and how calm/excited (i.e., arousal) participants indicated feeling in response to pleasant, unpleasant, and neutral stimuli (see Figure 1). Overall, patients and controls did not differ in magnitude of negative emotion to unpleasant stimuli, but patients reported more negative emotion to neutral and pleasant stimuli than controls (see Table 2 for ANOVA results). There was also no evidence for diminished hedonic capacity in schizophrenia, as there were no group differences in self-reported positive emotion or arousal to pleasant stimuli (see Table 2). Thus, results are comparable to past studies when standard analyses are applied.
Figure 1. Standard Ratings of Positive Emotion, Negative Emotion, and Arousal.
Note. A = How negative?; B = How positive?; C = Arousal. SZ = Schizophrenia; CN = Control. Values reflect means and error bars indicate standard error of the mean.
Table 2.
ANOVAs for schizophrenia and control group differences for standard analyses of valence and arousal
| Condition | Within-Subjects | Between-Subjects | Interaction | Post Hoc |
|---|---|---|---|---|
| How negative? | F (1, 134) = 1600.7*** | F (1, 134) = 21.2*** | F (1, 134) = 9.59** | SZ > CN neutral, pleasant; SZ = CN unpleasant |
| How positive? | F (1, 134) = 912.2*** | F (1, 134) = 2.78 | F (1, 134) = 9.75*** | SZ > CN neutral, unpleasant; SZ = CN pleasant |
| Arousal | F (1, 134) = 115.7*** | F (1, 134) = 0.02 | F (1, 134) = 7.32** | SZ < CN unpleasant; SZ = CN pleasant, neutral |
Note. SZ = schizophrenia; CN = control;
p < 0.05,
, p < 0.01,
p < 0.001;
Positivity Offset and Negativity Bias Analyses
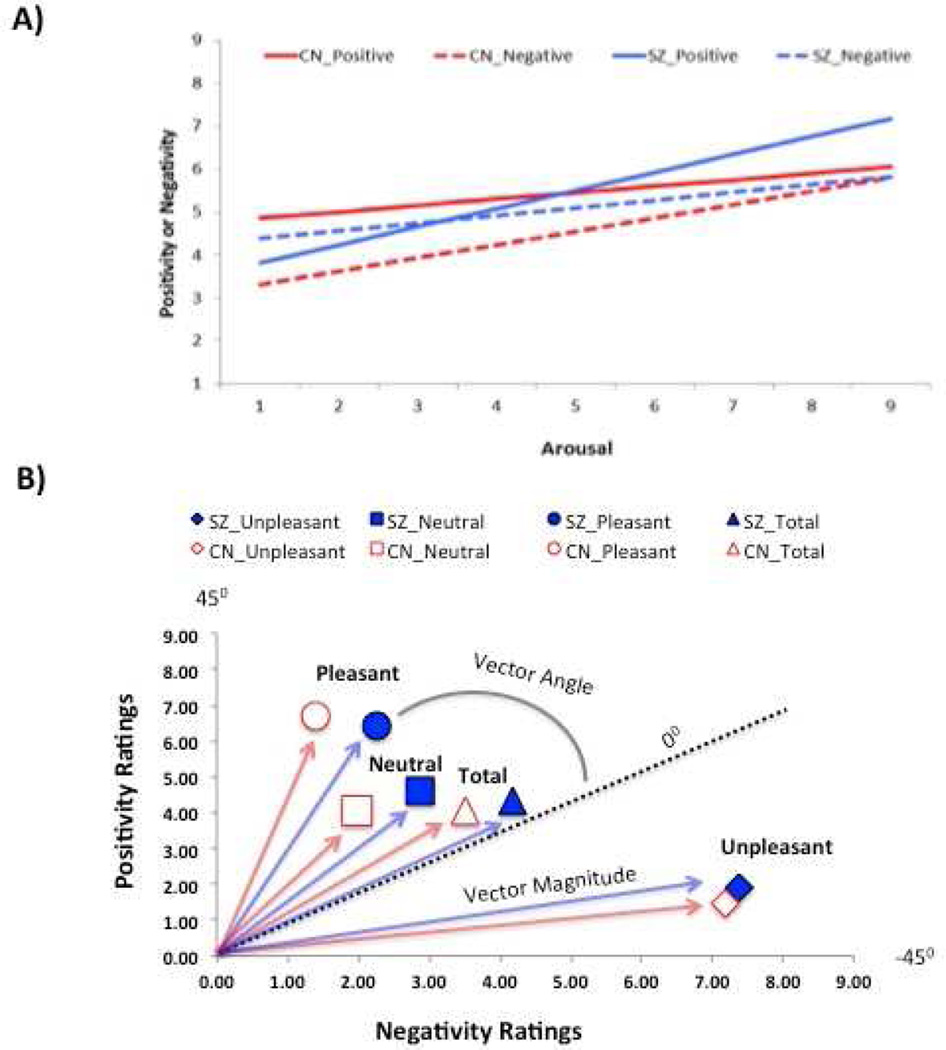
The positivity offset and negativity bias are depicted as regression lines in Figure 2A. Controls demonstrated the prototypical positivity offset, with a significantly higher intercept for positivity than negativity (positive intercept = 4.7, negative = 3.0, t = 2.33, p < 0.03). Patients failed to exhibit the typical positivity offset, instead displaying a trend toward a higher intercept for the negativity than positivity function (positive intercept = 3.12, negative = 4.2, t = 1.69, p = 0.09). Additionally, the intercept value of patients was significantly lower than controls for the positive function (t = 2.13, p < 0.04); however, there was no group difference in intercept for the negative function (t = 1.22, p = 0.22).
Figure 2. Positivity Offset, Negativity Bias, and Co-Activation.
Note. CN = control; SZ = schizophrenia. A) The positivity offset and negativity bias as seen in regression lines predicting positivity and negativity ratings from mean arousal. The X axis represents affective input, whereas the Y axis represents affective output. The positivity offset is reflected in a higher intercept for positive than negative output, and negativity bias reflected in steeper slope for negative than positive output. B) Co-Activation: Bivariate representation of positivity and negativity ratings in evaluative space using a two-dimensional Cartesian coordinate system, with negativity ratings on the X-axis, positivity ratings on the Y-axis, and the hypotenuse reflecting vector magnitude.
Most importantly, groups also differed in the positivity offset difference score (positive intercept – negative intercept) that was calculated for each individual subject, F (1, 134) = 16.68, p < 0.001, Cohen’s d = 0.71. (Schizophrenia: M = 1.28, SD = 5.01; Control: M = 4.98, SD = 5.53). Thus, patients display a reduced positivity offset.
Controls demonstrated the negativity bias, with numerically higher slope for the negative than positive function (Negative = 0.31, Positive = 0.15), although this effect was not statistically significant (t = 1.1, p = 0.26). In contrast, patients had significantly higher slope values for the positive (0.48) than negative (0.18) function (t = 2.46, p < 0.02). Patients also had significantly higher slope for positive emotion than controls (t = 2.1, p < 0.05), indicating intact hedonic capacity at increasing levels of affective input in schizophrenia. However, groups did not differ in slope for negativity (t = 0.25, p = 0.80), inconsistent with the hypothesis of greater negativity bias in schizophrenia.
Co-activation: Vector Angle and Magnitude Analyses
Figure 2B depicts mean positivity and negativity ratings within multidimensional evaluative space for pleasant, unpleasant, and neutral stimuli. These values formed the basis of the vector angle and magnitude calculations. Patients had significantly higher vector magnitude total scores than controls, F (1, 134) = 4.12, p < 0.05. Significant differences in vector magnitude were also observed for unpleasant, F (1, 134) = 4.82, p < 0.03, and neutral, F (1, 134) = 15.63, p < 0.001, but not pleasant stimuli, F (1, 134) = 1.33, p = 0.25.
Compared to controls, patients had lower vector angle scores for all stimuli, F (1, 134) = 9.28, p < 0.01, unpleasant stimuli, F (1, 134) = 6.17, p < 0.02, neutral stimuli, F (1, 134) = 4.13, p < 0.05, and pleasant stimuli, F (1, 134) = 31.36, p < 0.001.
Overall, results indicate that patients have greater co-activation of positive and negative emotions than controls, as indicated by higher vector magnitude scores and vector angle scores that fell near zero when examined for all stimuli.
Regression Analyses Predicting Anhedonia
The positivity offset (positive function intercept – negative function intercept), negativity bias (negative function slope), total vector angle, and total vector magnitude scores were used in two linear multiple regression analyses to predict anhedonia. The first analysis used the average of the Chapman physical and social anhedonia scores as the dependent variable in the schizophrenia sample. The overall model was significant (F = 6.13, p < 0.01), accounting for 25% of the variance in Chapman scale anhedonia scores. Two of the 4 predictors were significant (positivity offset: p < 0.05; vector magnitude: p < 0.05). In contrast, the model was not statistically significant in controls (F = 0.59), with the 4 variables accounting for only 7% of variance in Chapman scale scores.
The second linear regression analysis, which was conducted only in the schizophrenia group, used the BNSS anhedonia subscale as the dependent variable. There was a trend toward significance in the overall model (F = 1.79, p = 0.09), with the predictor variables accounting for 9% of variance in BNSS anhedonia. Positivity offset (p < 0.05) and vector magnitude (p < 0.05) were significant predictors of BNSS anhedonia in schizophrenia patients2.
Discussion
Early clinical descriptions of schizophrenia emphasized that patients were anhedonic in that they displayed a diminished capacity to experience pleasure (Kraepelin, 1919; Bleuler, 1911; Rado, 1953). However, the field has recently moved away from this view following consistent empirical evidence that schizophrenia patients report levels of positive emotion and arousal that are comparable to controls when exposed to pleasant stimuli (Kring & Moran, 2008; Strauss & Gold, 2012; Cohen & Minor, 2010; Gard et al., 2007; Gold et al., 2008; Barch & Dowd, 2010; Kring & Ellis, 2013; Cohen et al., 2011). Consistent with the majority of published studies (Cohen & Minor, 2010; Llerena, Strauss, Cohen, 2012), we also found that schizophrenia patients did not differ from controls in self-reported positive emotion or arousal to pleasant stimuli. We did, however, find evidence for several abnormalities in state emotional experience when data were considered from the Evaluative Space Model (Cacioppo & Berntson, 1994), and these abnormalities significantly predicted anhedonia assessed via trait questionnaires and a negative symptom clinical interview.
The first abnormality consisted of a reduction in the normative positivity offset. Specifically, schizophrenia patients had lower intercept values for positive emotion than controls indicating that patients experience reductions in positive emotion output at lower levels of stimulus input. Schizophrenia patients also displayed a reduction in the positive/negative intercept ratio score, indicating an imbalance in the level of positive to negative emotion at lower levels of affective input. At first glance, the reduction in positivity offset appears at odds with evidence for a lack of group differences in self-reported positive emotion to pleasant stimuli that has consistently been observed in the literature and was also observed here. However, this discrepancy can be reconciled when one considers that patients also had significantly greater slope values for positive emotion than controls. These findings suggest that patients have lower positive emotion than controls when affective stimulus input is low. However, as input increases, patients are able to ramp up their level of positive emotion normally, or even to a greater extent than controls. The more simplistic analysis of how positive patients feel in relation to pleasant stimuli may therefore mask the important observation that a hedonic deficit exists only at lower levels of affective input, but this deficit is overcome when affective input increases. These findings are inconsistent with the straightforward notion of diminished hedonic capacity in schizophrenia, which would be indicated by a reduction in positive emotion for stimuli with high affective input (i.e., those that tax maximal affective response). Hedonic abnormalities only appear to emerge in schizophrenia at low levels of affective input. The absence of a positivity offset may explain why schizophrenia patients often fail to seek out rewarding activities in real-life contexts where the majority of time points have low or absent affective input (Gard et al., 2007; Oorschot et al., 2013; Myin-Germeys et al., 2000; Gard et al., 2014). Specifically, in more neutral contexts, the failure to reach a minimum threshold of positive emotion, as well as lower ratio of positive to negative emotion, may reduce the likelihood of performing exploratory behaviors and approaching novel or rewarding stimuli in a neutral environment.
A second abnormality involved increased co-activation of positive and negative emotion in schizophrenia. Greater intensity of co-activation has been inferred in past schizophrenia studies, which provided evidence for incongruency of emotional self-report in patients (i.e., greater negative emotion to pleasant stimuli and greater positive emotion to unpleasant stimuli) (Tremeau et al., 2010; Sanchez et al., 2014; Strauss & Herbener, 2011; Docherty et al., 2014). However, we provided the first formal test of co-activation by applying vector-based equations developed in relation to the Evaluative Space Model (Norman et al., 2011). Increased co-activation was indicated by: 1) greater total vector magnitude scores in patients than controls, signifying higher combined total intensity of co-activated positive and negative emotion; and 2) mean total vector angle scores that fell around 0 in the schizophrenia group, signifying a relative balance of activated positive and negative emotion. In healthy individuals, periods of co-activation are thought to be highly aversive, unstable, and short-lived, having detrimental effects on the initiation of motivated behaviors (Larsen, McGraw, Cacioppo, 2001; Norris et al., 2010). In schizophrenia patients, co-activations could be expected to have a similar effect, resulting in a blurred affective state that is maladaptive and not pure enough to motivate the initiation of approach behaviors.
The third major finding involved the vector angle analyses, which consider the relative balance of positive and negative emotion activated in response to each stimulus. Results indicated lower vector angle values in patients, signifying a global shift in evaluative space toward less positivity for pleasant and neutral stimuli. These findings are consistent with results from a meta-analysis of laboratory-based studies, which found that even though schizophrenia patients do not report decreased positive emotion to pleasant stimuli, they do experience elevated negative emotion to pleasant and neutral stimuli at a medium effect size (Cohen & Minor, 2010). Our vector angle findings extend this prior work by indicating that the overall balance between positive and negative emotion still achieves a net positive value in schizophrenia. However, the level of negative emotion experienced may be sufficiently high enough to cancel out concurrently experienced positive emotion, resulting in a global affective state that fails to swing the motivational pendulum high enough to influence behavior.
Certain limitations should be considered when interpreting these results. First, although the IAPS stimuli form the backbone of much of the affective science research, they come with some limitations when used in this context (e.g., norms based on college students may not be comparable for middle-aged community members or persons with lower education, ecological validity, etc). Future studies should replicate these results using alternative stimulus types and Ecological Momentary Assessment. Second, although the Evaluative Space Model, has used stimulus arousal as a proxy for the level of input into the affective system, it is unclear whether this is valid in schizophrenia. We suspect it is, as there is a large body of research indicting that increases in stimulus arousal are associated with increased activation of biological processes (e.g., autonomic, neurophysiological) that lead to the initiativon of appetitive and defensive motivated behaviors (Bradley et al., 2001). Additionally, we did not find an overall group difference in self-reported arousal, suggesting that arousal may indeed be a valid representation of the level of input into the affective system. Third, the Evaluative Space Model was examined only in relation to self-report in the current study. Subjective experience represents only one component of emotion response and other methods of measurement should be obtained concurrently in future studies (e.g., psychophysiology, neuroimaging). This may be particularly important in schizophrenia because abnormalities in affective system response may be detected using methods (e.g., neuroimaging, peripheral psychophysiology) that could diverge from self-report. At present, the level of coherence among different types of emotional response is not known in schizophrenia, and it is therefore impossible to gauge whether or how responses in these other channels might relate to the positivity offset or affective co-activation. Finally, the mechanism of co-activation cannot be determined via the current methods alone. The Evaluative Space Model points to two possibilities. One possibility is that patients are more likely than controls to engage in “parallel evaluative processing” (i.e., co-activation is achieved by attending to positive and negative features of a stimulus simultaneously, e.g., something that is bitter sweet). Alternatively, co-activation may result from “oscillation” (i.e., switching between positive and negative stimuli so frequently that it results in sustained activation of both emotions). Rapid oscillation between positive and negative states may operate like a low-pass filter, removing high frequency activity (i.e., the uniquely experienced states of positive and negative emotion), leaving only a blurred affective state that is a less pure and mixed product of the two.
Despite these limitations, findings provide a promising new direction toward clarifying the nature of emotional experience in schizophrenia. Prior conclusions that schizophrenia patients do not display a reduced hedonic capacity appear to be correct. Our findings indicate that schizophrenia patients do not appear to be anhedonic, at least not in terms of how the symptom has traditionally been defined. However, it would be incorrect to state that hedonic response is fully normal in schizophrenia. Our findings suggest that schizophrenia is characterized by a complex pattern of affective abnormalities that can best be described as: 1) increased co-activation of positive and negative emotions that lower the overall net hedonic value of stimuli, and 2) an imbalance in the ratio of positive to negative emotion at lower levels of affective input (i.e., reduction in positivity offset). Furthermore, the group difference in the positivity offset (Cohen’s d = 0.71) and proportion of variance accounted for in the Chapman anhedonia scale scores (25%) were both medium to large effects. It is therefore possible that the positivity offset and co-activation abnormalities are core features of schizophrenia that track with negative symptom severity. Future studies should determine whether these variables are associated with other aspects of reward processing (e.g., value representation, reinforcement learning, effort-cost computation) that are core components of current etiological models of anhedonia and avolition (Barch & Dowd, 2010; Gold et al., 2008; Kring & Barch, 2014). Finally, our results point to an important caveat-the positivity offset abnormality is present in contexts with low or absent affective input. The specificity of this finding has important implications for psychosocial treatments for negative symptoms, suggesting that interventions should explicitly aim to increase positive emotion and lower negative emotion in these contexts/environments. Mobile technology and Ecological Momentary Intervention may offer a novel means of achieving this purpose. These tools could be used to prompt participants to engage in planned pleasurable activities more frequently or to anticipate/remember positive events in contexts that lack inherent affective input. Additionally, several apps now exist that allow for geotracking and accelerometry monitoring, which may be able to serve as alerts for when activity level has become too low, the number of contexts traversed in a day too limited, or patients are not performing a scheduled activity at a specific location. Timing these interventions to take place within low affective input contexts seems like it would be the most critical aspect of treating motivational deficits based on the nature of the emotional experience abnormalities observed in the current study. Finally, although future studies are needed to replicate these findings and determine their reliability, the evaluative space model (Cacioppo & Berntson, 1994) may be a promising theoretical perspective for exploring emotional experience in schizophrenia. The well-validated quantitative methods pioneered by Ito and Cacioppo (2005) and Norman et al. (2011) offer new tools for assessing emotional experience in schizophrenia during laboratory and experience sampling studies.
Acknowledgments
We would like to thank the patients and staff at the Maryland Psychiatric Research Center for their participation in this research and facilitation of the study. In particular, we would like to thank Lauren Catalano and Adam Culbreth for study coordination and research duties.
Funding and Disclosures
Research was supported by NIMH grant K23MH092530 to Dr. Strauss. Dr. Strauss is one of the original developers of the Brief Negative Symptom Scale (BNSS) and receives royalties and consultation fees from ProPhase LLC in connection with commercial use of the BNSS and other professional activities. Dr. Gold receives royalty payments from the BACS and he has been a consultant to Amgen, Hoffman LaRoche, Pfizer, Merck, Astra Zenaca, Solvay, Takeda, Lundbeck, and Glaxo Smith Kline.
Footnotes
There were no Experiment × Group or Experiment × Group × Stimulus Valence interactions for any of the main analyses (i.e., vector angle, vector magnitude, positivity offset, negativity bias, how positive reports, how negative reports, arousal reports). Demographics and patient symptom severity were also comparable across the 3 Experiments. As such, combining data across the 3 studies seemed justifiable, despite use of different numbers of stimuli in each experiment and different IAPS images.
ANOVA also indicated that patients with high BNSS ratings had lower positivity offset scores than those with low BNSS ratings, F = 4.1, p < 0.05 (HIGH: M = −0.24 (5.7); LOW: M = 3.2 (4.5)).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: Author; 2000. 4, text revised ed. [Google Scholar]
- Ashare RL, Norris CJ, Wileyto EP, Cacioppo JT, Strasser AA. Individual differences in positivity offset and negativity bias: Gender-specific associations with two serotonin receptor genes. Personality and individual differences. 2013;55(5):469–473. doi: 10.1016/j.paid.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal–striatal interactions. Schizophrenia Bulletin. 2010;36(5):919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox or the group of Schizophrenias. 1950 [PubMed] [Google Scholar]
- Boucher J, Osgood CE. The pollyanna hypothesis. Journal of Verbal Learning & Verbal Behavior. 1969;8:1–8. [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276. [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space: a critical review, with emphasis on the separability of positive and negative substrates. Psychological bulletin. 1994;115(3):401. [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, Ito TA. The psychophysiology of emotion. In: Lewis R, Haviland-Jones JM, editors. The Handbook of Emotion. 2nd. New York: Guilford Press; 2000. pp. 173–191. [Google Scholar]
- Carretié L, Martín-Loeches M, Hinojosa JA, Mercado F. Emotion and attention interaction studied through event-related potentials. Journal of Cognitive Neuroscience. 2001a;13:1109–1128. doi: 10.1162/089892901753294400. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophrenia bulletin. 2010;36(1):143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Najolia GM, Brown LA, Minor KS. The state-trait disjunction of anhedonia in schizophrenia: potential affective, cognitive and social-based mechanisms. Clinical psychology review. 2011;31(3):440–448. doi: 10.1016/j.cpr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Van Bavel JJ, Johnsen IR. Affective flexibility: evaluative processing goals shape amygdala activity. Psychological Science. 2008;19:152–160. doi: 10.1111/j.1467-9280.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. Journal of abnormal psychology. 1976;85(4):374. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Docherty AR, Sponheim SR, Kerns JG. Further examination of ambivalence in relation to the schizophrenia spectrum. Schizophrenia research. 2014;158(1):261–263. doi: 10.1016/j.schres.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ, Chapman JP, Mishlove M. The revised social anhedonia scale [Unpublished Test] Madison: University of Wisconsin; 1982. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structural Clinical Interview for DSM-IV Axis I Disorders (SCID-IV) New York: New York State Psychiatric Institute, Biometrics Research; 1997. [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia research. 2007;93(1):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, Vinogradov S. Do people with schizophrenia have difficulty anticipating pleasure, engaging in effortful behavior, or both? Journal of abnormal psychology. 2014;123(4):771. doi: 10.1037/abn0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophrenia bulletin. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan JK, Connolly M, Buchanan A, Hoxha D, Rosebrock L, Cacioppo J, et al. Neural substrates of negativity bias in women with and without major depression. Biological Psychology. 2015;109:184–191. doi: 10.1016/j.biopsycho.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Gollan JK, Hoxha D, Hunnicutt-Ferguson K, Norris CJ, Rosebrock L, Sankin L, Cacioppo J. Twice the negativity bias and half the positivity offset: Evaluative responses to emotional information in depression. Journal of behavior therapy and experimental psychiatry. 2016;52:166–170. doi: 10.1016/j.jbtep.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harinck F, Van Dijk E, Van Beest I, Mersmann P. When gains loom larger than losses: reversed loss aversion for small amounts of money. Psychological Science. 2007;18:1099–1105. doi: 10.1111/j.1467-9280.2007.02031.x. [DOI] [PubMed] [Google Scholar]
- Hawk AB, Carpenter WT, Strauss JS. Diagnostic criteria and five-year outcome in schizophrenia. A report from the International Pilot Study of schizophrenia. Archives of general psychiatry. 1975;32(3):343–347. doi: 10.1001/archpsyc.1975.01760210077005. [DOI] [PubMed] [Google Scholar]
- Ito T, Cacioppo J. Variations on a human universal: Individual differences in positivity offset and negativity bias. Cognition & Emotion. 2005;19(1):1–26. [Google Scholar]
- Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: the negativity bias in evaluative categorizations. Journal of Personality and Social Psychology. 1998;75:887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Choices, values, and frames. American Psychologist. 1984;39:341–350. [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, Marder SR. The brief negative symptom scale: psychometric properties. Schizophrenia bulletin. 2011;37(2):300–305. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox and paraphrenia. Krieger Publishing Company; 1971. [Google Scholar]
- Kring AM, Elis O. Emotion deficits in people with schizophrenia. Annual review of clinical psychology. 2013;9:409–433. doi: 10.1146/annurev-clinpsy-050212-185538. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophrenia bulletin. 2008;34(5):819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- Larsen JT, McGraw AP, Cacioppo JT. Can people feel happy and sad at the same time? Journal of personality and social psychology. 2001;81(4):684. [PubMed] [Google Scholar]
- Larsen JT, Norris CJ, McGraw AP, Hawkley LC, Cacioppo JT. The evaluative space grid: a single-item measure of positivity and negativity. Cognition and Emotion. 2009;23(3):453–480. [Google Scholar]
- Llerena K, Strauss GP, Cohen AS. Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophrenia research. 2012;142(1):65–70. doi: 10.1016/j.schres.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I, Delespaul PA. Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophrenia Bulletin. 2000;26(4):847–854. doi: 10.1093/oxfordjournals.schbul.a033499. [DOI] [PubMed] [Google Scholar]
- Neta M, Norris CJ, Whalen PJ. Corrugator muscle responses are associated with individual differences in positivity-negativity bias. Emotion. 2009;9:640–648. doi: 10.1037/a0016819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CJ, Larsen JT, Crawford LE, Cacioppo JT. Better (or worse) for some than others: individual differences in the positivity offset and negativity bias. Journal of Research in Personality. 2011;45(1):100–111. [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Karelina K, Malarkey WB, DeVries AC, Berntson GG. Selective influences of oxytocin on the evaluative processing of social stimuli. Journal of Psychopharmacology. 2011;25(10):1313–1319. doi: 10.1177/0269881110367452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CJ, Gollan J, Berntson GG, Cacioppo JT. The current status of research on the structure of evaluative space. Biological psychology. 2010;84(3):422–436. doi: 10.1016/j.biopsycho.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oorschot M, Lataster T, Thewissen V, Lardinois M, Wichers M, van Os J, Myin-Germeys I. Emotional experience in negative symptoms of schizophrenia—no evidence for a generalized hedonic deficit. Schizophrenia bulletin. 39:217–225. doi: 10.1093/schbul/sbr137. (20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological reports. 1962;10(3):799–812. [Google Scholar]
- Pfohl BM, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality. 1. American Psychiatric Publishing, Inc.; 1997. [Google Scholar]
- Rado S. Dynamics and classification of disordered behavior. American Journal of Psychiatry. 1953;110(6):406–416. doi: 10.1176/ajp.110.6.406. [DOI] [PubMed] [Google Scholar]
- Sanchez AH, Lavaysse LM, Starr JN, Gard DE. Daily life evidence of environment-incongruent emotion in schizophrenia. Psychiatry research. 2014;220(1):89–95. doi: 10.1016/j.psychres.2014.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears DO. The person-positivity bias. Journal of Personality and Social Psychology. 1983;44:233–250. [Google Scholar]
- Smith NK, Larsen JT, Chartrand TL, Cacioppo JT, Katafiasz HA, Moran KE. Being bad isn't always good: affective context moderates the attention bias toward negative information. Journal of Personality and Social Psychology. 2006;90(2):210–220. doi: 10.1037/0022-3514.90.2.210. [DOI] [PubMed] [Google Scholar]
- Strauss GP. Anhedonia: A Comprehensive Handbook Volume II. Netherlands: Springer; 2014. Anhedonia in schizophrenia: a deficit in translating reward information into motivated behavior; pp. 125–156. [Google Scholar]
- Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. American Journal of Psychiatry. 2012;169(4):364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Herbener ES. Patterns of emotional experience in schizophrenia: differences in emotional response to visual stimuli are associated with clinical presentation and functional outcome. Schizophrenia research. 2011;128(1):117–123. doi: 10.1016/j.schres.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Asymmetrical effects of positive and negative events: themobilization-minimization hypothesis. Psychological Bulletin. 1991;110:67–85. doi: 10.1037/0033-2909.110.1.67. [DOI] [PubMed] [Google Scholar]
- Trémeau F, Antonius D, Cacioppo JT, Ziwich R, Jalbrzikowski M, Saccente E, Javitt D. In support of Bleuler: objective evidence for increased affective ambivalence in schizophrenia based upon evocative testing. Schizophrenia research. 2009;107(2):223–231. doi: 10.1016/j.schres.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Ursu S, Kring AM, Gard MG, Minzenberg MJ, Yoon JH, Ragland JD, Carter CS. Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. American Journal of Psychiatry. 2011;168:276–285. doi: 10.1176/appi.ajp.2010.09081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajonc RB. Attitudinal effects of mere exposure. Journal of Personality and Social Psychology. 1968;9:1–27. [Google Scholar]