Abstract
Background
The aim of this study was to study the effects of 1-alpha,25-dihydroxy-cholecalcifero (1,25(OH)2D3) on liver fibrosis and the generation of Th17 cells in vivo and in vitro.
Material/Methods
Thirty C57 mice were randomly divided into control, model, and treatment groups. Hepatic fibrosis was induced by subcutaneous injection of CCl4. Liver fibrosis condition was evaluated through pathological inspection and blood biochemical examination of liver function. Immunohistochemical assays were used to detect the expression of α-SMA, TGF-β, and collagen I to observe hepatic stellate cell activation level. Flow cytometry, ELISA, and RT-PCR were performed to explore the association between 1,25(OH)2D3 and Th17 cell differentiation.
Results
Collagen I, TGF-β, and α-SMA were decreased after 1,25(OH)2D3 treatment. Consistently, RORγt mRNA and the rate of Th17 cells was significantly reduced after 1,25(OH)2D3 treatment. In vitro, the proportion of Th17 cells was also obviously reduced in the 1,25(OH)2D3 group, and mRNA levels of IL-17A, IL-22, RORγt, and RORα were significantly decrease in the 1,25(OH)2D3 group compared to the control group.
Conclusions
Treatment with 1,25(OH)2D3 can alleviate the damage caused by liver fibrosis. Experiments in vivo and in vitro showed that 1,25(OH)2D3 treatment deceased the rates of Th1 and Th17 cells and increased the rate of Th2 cells. The level of IL-17A, IL-22 and IFN-γ were decreased, while the level of IL-4 was increased by the treatment of 1,25(OH)2D3.
MeSH Keywords: Calcitriol; Hypertension, Portal; Liver Cirrhosis; T-Lymphocytes, Helper-Inducer
Background
1-alpha,25-dihydroxy-cholecalcifero (1,25(OH)2D3), as a steroid hormone, plays a physiological function through vitamin D receptor (VDR) binding to tissues and organs. In addition to calcium and phosphorus metabolism, 1,25(OH)2D3 plays a significant role in the regulation of various biological processes (e.g., immune regulation, cell proliferation and differentiation, and anti-fibrosis) [1]. The liver and kidney play a vital role in activating vitamin D, and studies have shown that there is a correlation between vitamin D and pathogenesis and treatment of chronic liver and kidney disease. Recently, studies have shown vitamin D has an effect on anti-fibrosis [2]. 1,25(OH)2D3 inhibits the proliferation of immune cells and the production of immunoglobulin, and can prevent precursor B-cells from differentiating into plasma cells [3]. In addition, 1,25(OH)2D3 inhibits the proliferation of T cells, and inhibits the expression ability of interferon and IL-2 in Th1 cells, and activates the expression of IL-17 and IL-22 in macrophages and Th17 cells [4,5]. 1,25(OH)2D3 also has an anti-inflammatory effect, inhibits infiltration of inflammatory factors, and reduces damage to renal tubular interstitial by inflammatory response [6]. In in vitro experiments, 1,25(OH)2D3 inhibits activation of lung fibroblasts and epithelial cells and conversion between type II alveolar epithelial and interstitial cells induced by TGF-β 1, thus preventing a large number of mesenchymal cells from proliferation and collagen deposition [7]. Takeda et al. [8] found that oral administration of 1,25(OH)2D3 could prevent the development of atherosclerosis disease by changing the function and differentiation of dendritic cells and regulatory T cells (Treg cells). Tang et al. [9] found that 1,25(OH)2D3 inhibited innate immune response by inhibiting Th17 response, including the ability to induce Th17 cells supported by dendritic cells, CD4+T cells differentiating into Th17 cells, and Th17 cells ability to express IL-17. Chang et al. [10] demonstrated that 1,25(OH)2D3 inhibited differentiation of Th17 and Treg cells which could express IL-17 through the signal of vitamin D3 receptor in vitro. CTC (carbon tetrachloride, CCl4) causes degeneration and necrosis of liver cells by the liver cytochrome 2E1 (CYP2E1) [11], while fat-storing cells are activated, which releases type IV collagenase, degrades type IV collagen, synthesizes and secretes type I collagen, and promotes the development of liver fibrosis [12]. In this study, a liver fibrosis model was constructed using CCl4. Pathological changes and changes in CD4+T cell subsets were detected to explore the treatment of 1,25(OH)2D3 in liver fibrosis and the inhibition of 1,25(OH)2D3 in Th17 cell differentiation.
Material and Methods
Animals
Thirty healthy eight-week-old C57 mice were purchased from Changzhou Cavens Experimental Animals Ltd., People’s Republic of China. The mice were kept under standard conditions of boarding and feeding with free access to water. All animal experiments were approved by the Ethical Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, People’s Republic of China).
Mice groups and treatments
Thirty C57 mice were randomly divided into three groups (n=10 for each group). Group 1 was the control group mice that received 100 uL normal saline (NS) by intraperitoneal injection twice weekly for eight weeks. Group 2 was the fibrosis group mice that received 100 uL CCl4 (20% in olive oil) intraperitoneal two times a week for eight weeks. Group 3 was the 1,25(OH)2D3 treatment group mice that received the same treatment as group 2 plus daily intraperitoneal injections of 100 uL of 1,25(OH)2D3 (5 ug/kg). At the end of the eighth week the mice were sacrificed, 1 mL blood was collected by retro-orbital artery. The blood sample was centrifuged at 3,000 rpm for 20 minutes; serum was collected in aliquots and kept frozen at −80°C for cytokines determination. The liver was quickly weighed and divided into two portions: one portion was embedded with embedding medium frozen in liquid nitrogen tank for frozen sections, and the other was immediately frozen in liquid nitrogen, and then transferred to a −8°C freezer. Liver tissue samples were finally used for real-time polymerase chain reaction (RT-PCR). During the injection period, the concept of 3Rs (replacement, reduction, refinement) was considered.
Histopathological analysis
Tissue sections were stained with hematoxylin and eosin solutions (H&E) and Sirius-red staining to observe pathological changes in liver tissue.
Liver function and cytokine levels detection
Using the kit instructions, cytokines (IL-4, IL-17A IL-22, and IFNγ) were detected by ELISA. Liver function was detected according to the kit instructions (Jianchen Nanjin).
Immunohistochemistry
Specimens were fixed in 4% paraformaldehyde for 10 minutes. After treatment with 3% hydrogen peroxide in methanol to quench the endogenous peroxidase activity, the sections were submerged into citrate buffer and high-pressure boiled for antigenic retrieval, followed by incubation with 1% bovine serum albumin to block the nonspecific binding. Anti-α-SMA, anti-TGF-β, and anti-collagen I (Abcam) were incubated with the sections overnight at 4°C, respectively. After washing with PBS, the tissue sections were incubated with CY3-labeled secondary antibody. Then after washing, the tissue sections were incubated with 3,3-diaminobenzidin (DAB) and counterstained with hematoxylin, dehydrated, and mounted. The sections were reviewed and scored independently by two observers, based on both the proportion of positively stained tumor cells and the intensity of staining.
Hepatosplenic T-cell lymphocyte subsets analyze
Liver and spleen lymphocytes were prepared, respectively. They were detected by flow cytometry after incubation to identify phenotype.
Inducing Th17 cell differentiation
Initial CD4+T cells were prepared by MACS (Press kit instructions). CD4+T cells were cultured with 10% fetal bovine serum-containing RPMI1640, then we added anti-human CD3/CD28 mAb, anti-human IL-2, IFN-γ monoclonal antibody, and recombinant human cytokines (TGF-β, IL-6, IL-1β). CD4 + T cells were divided into four groups: control group, low dose group (1,25(OH)2D3) at 10−9 mol/L), middle dose group (1,25(OH)2D3 at 10−8 mol/L), and high dose group (1,25(OH)2D3 at 10−7 mol/L). The cells were collected five days later, and T-cell subsets of spleen lymphocytes were analyzed by flow cytometry.
Real-time polymerase chain reaction (RT-PCR)
Total RNAs from the liver tissue samples were isolated using the TRIzol reagent; cDNAs were synthesized using reverse transcription kit (Thermo Fisher Scientific). RT PCR was performed with 2 uL cDNA per reaction using 12.5 uL of SYBR Green QPCR Mix containing 1 uL of specific primers in the RT-PCR Detection System. The data were analyzed by relative software (ABI Prism 7500 SDS Software).
Statistical analysis
Statistics were done using relative software (Origin 6.1). Measurement data in normal distribution were expressed as mean ± standard deviation (SD). The t-test was used to compare between groups. Statistical significance was set at a value of p<0.05; extremely statistical significance was set at a value of p<0.01.
Results
1,25(OH)2D3 alleviated liver fibrosis in mice
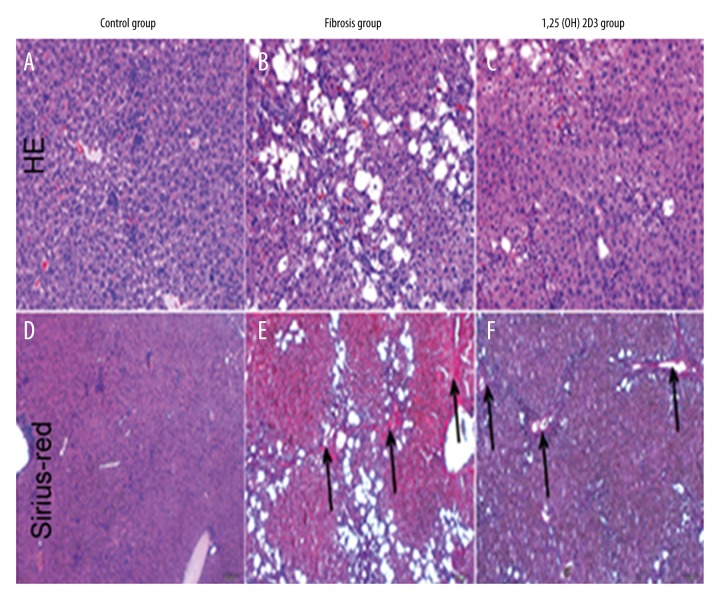
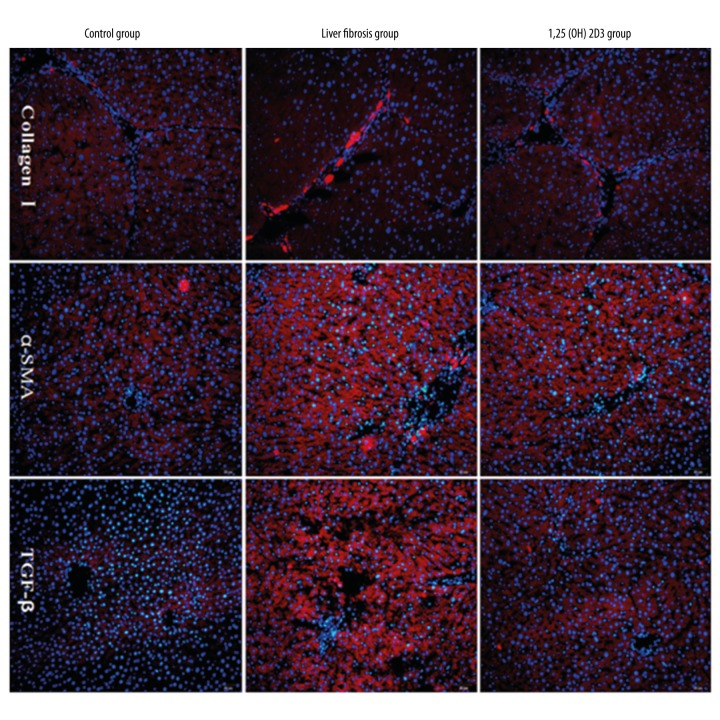
H&E staining showed that hepatocytes in the control group were normal and had distinct cytoplasm and nucleus, uniform size distribution, and no visible fibrosis tissue. While the nucleus boundary was not clear in the model group, and a large number of vacuoles and fibrosis tissue were identified. The recovery of cells in the 1,25(OH)2D3 treatment group was good, with a small amount of vacuoles and fibrosis tissue identified (Figure 1A–1C). Sirius-red staining showed that liver tissue in the control group was normal and there was no visible fibrosis, while a large amount of fibrosis tissue was identified in the model group (black arrow shows areas of fibrosis). The recovery of cells in the 1,25(OH)2D3 treatment group was good, with a small amount of fibrosis tissue identified (Figure 1D–1F). The indexes of liver function in serum are shown in Table 1. Compared with the control group, the levels of AST and TBIL significantly increased while the level of ALB was reduced in the liver fibrosis group with statistical significance (p<0.01). Compared with the liver fibrosis group, the levels of AST and TBIL significantly were reduced while the level of ALB increased in the 1,25(OH)2D3 group with statistical significance (p<0.01). These results indicated that liver fibrosis could lead into a decline of related liver function indexes, and 1,25(OH)2D3 significantly improved the structure of the liver tissue and liver fibrosis. Collagen I, TGF-β, and α-SMA were detected by immunofluorescence in liver tissue (Figure 2).Compared with the control group, expression of collagen I, TGF-β, and α-SMA increased significantly in the fibrosis group, which was significantly lower in the 1,25(OH)2D3 treatment group. These results showed that a large number of hepatic stellate cells (HSCs) were activated and the expression of the corresponding proteins was increased in liver fibrosis. After 1,25 (OH) 2D3 treatments, HSCs and the expression of the corresponding proteins were significantly reduced, but still higher than the control group.
Figure 1.
Pathological detection of liver in mice (A–C: H&E staining; D–F: Sirius-red staining, black arrow shown as fibrosis area).
Table 1.
The concentration of serum ALT, AST, ALB, and TBIL in mice.
| Group | ALT (U/L) | AST (U/L) | ALB (g/L) | TBIL (μmol/L) |
|---|---|---|---|---|
| Control group | 41.93±4.04 | 162.97±8.75 | 45.48±3.09 | 4.236±0.20 |
| Liver fibrosis group | 154.65±12.22** | 362.69±22.63** | 24.53±2.37** | 13.73±0.50** |
| 1,25(OH)2D3 group | 89.62±5.57## | 232.92±17.23## | 33.32±2.12## | 8.21±0.59## |
p<0.05 when compared with the normal group,
p<0.01;
p<0.05 when compared with the liver fibrosis group,
p<0.01.
Figure 2.
Immunofluorescence assay of collagen I, TGF-β, and α-SMA of liver in mice (blue: nuclear staining by Hoechst; red: protein expression).
The proportion of TH17 deceased after 1,25(OH)2D3 treatment in hepatic fibrosis
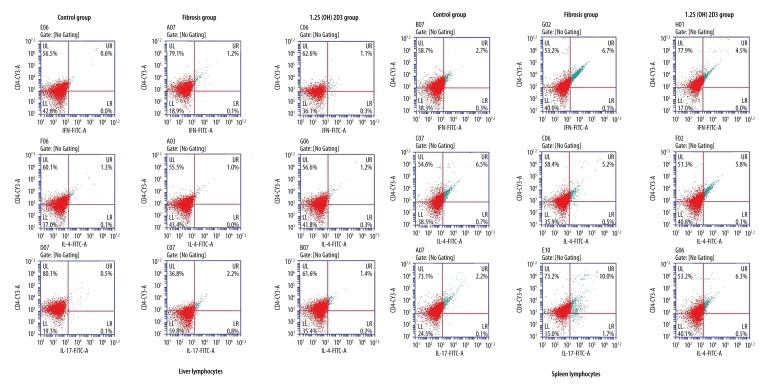
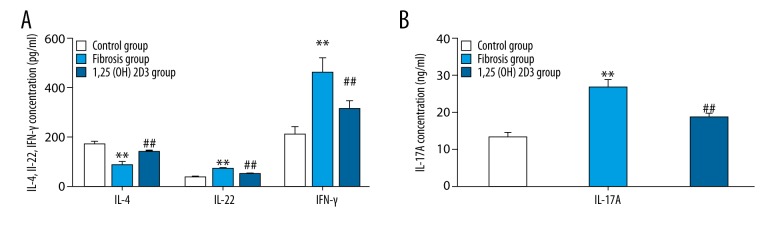
The liver and spleen lymphocytes were separated. Flow cytometry showed that the rates of Th1 cells and Th17 cells increased, whereas, the rate of Th2 cells decreased in the liver fibrosis group when compared with the control group, and 1,25(OH)2D3 treatment deceased the rates of Th1 cells and Th17 cells and increased the rate of Th2 cells when compared with the fibrosis group (CD4+IL-17+T cells were defined as Th17 cells, CD4+IFN-γ+T cells were defined as Th1 cells, CD4+IL-4+T cells were defined as Th2 cells, upper right quadrant was the ratio of the cell phenotypes) (Figure 3). The statistic results were shown in the Table 2. RT-PCR showed that the mRNA levels of RORγt (retinoid related orphan receptor gamma) and T-bet were significantly higher, whereas, GATA3 mRNA level was markedly lower in the liver fibrosis mice than those of control mice with statistical significance (p<0.01). The mRNA levels of RORγt and T-bet were significantly reduced, whereas, the GATA3 mRNA level was markedly increased in 1,25(OH)2D3 treatment mice compared with liver fibrosis mice (p<0.01) (Figure 4). ELISA (Figure 5) showed that liver fibrosis mice increased while 1,25(OH)2D3 treatment mice reduced IFNγ, IL-17A, and IL-22 concentration. On the contrary, IL-4 was reduced in liver fibrosis mice while increased in 1,25(OH)2D3 treatment mice. All differences between them are significantly (p<0.01).
Figure 3.
The percentage of each subgroup in CD4+T cells of liver and spleen lymphocytes in mice detected by flow cytometry instrument (CD4+ IL-17+ T cell was defined as Th17 cell, CD4+ IFN-γ+ T cell was defined as Th1 cell, CD4+ IL-4+ T was defined as Th2 cell).
Table 2.
The percentage of each subgroup in CD4+T cells of liver and spleen lymphocytes.
| Group | Liver lymphocytes (%) | Spleen lymphocytes (%) | ||||
|---|---|---|---|---|---|---|
| Th1 | Th2 | Th17 | Th1 | Th2 | Th17 | |
| Control group | 0.6 | 1.3 | 0.5 | 2.7 | 6.5 | 2.2 |
| Fibrosis group | 1.2** | 1.0* | 2.2** | 4.5** | 5.2** | 10.0** |
| 1,25(OH)2D3 group | 1.1# | 1.2# | 1.4## | 6.7## | 5.8## | 6.3## |
p<0.05 when compared with the control group,
p<0.01;
p<0.05 when compared with the liver fibrosis group,
p<0.01.
Figure 4.
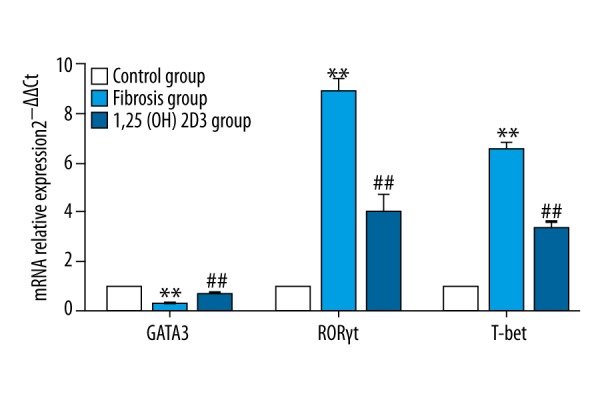
The expression of GATA3, RORγt, and T-bet mRNA in liver of mice (* means compared with control group, ** p<0.01; # means compared with hepatic fibrosis group, ## p<0.01).
Figure 5.
The concentration of cytokines in serum of mice, IL-4, IL-22 and IFN-γ was shown in A while IL-17A was shown in B (* means compared with control group, ** p<0.01; # means compared with hepatic fibrosis group, # p<0.05; ## p<0.01).
1,25(OH)2D3 inhibits differentiation of Initial CD4+T cells to TH17 cell in vitro
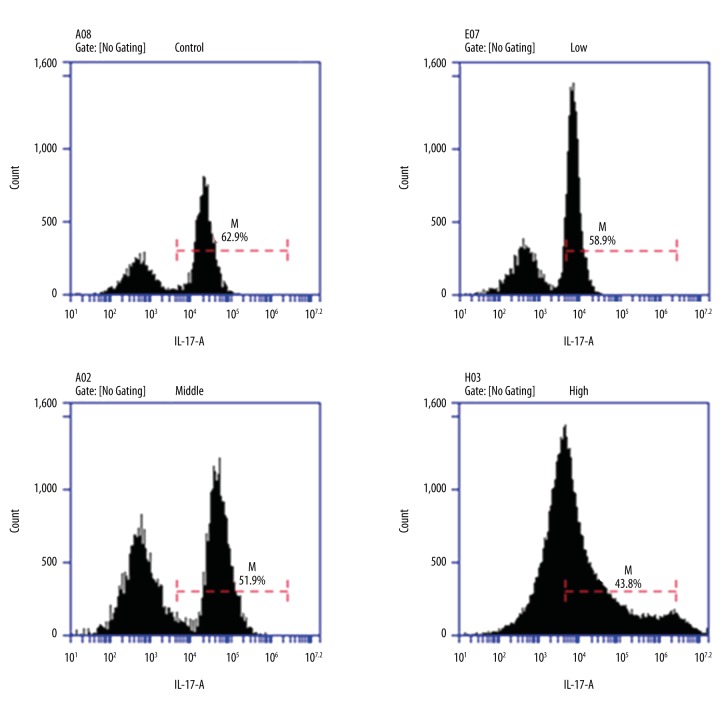
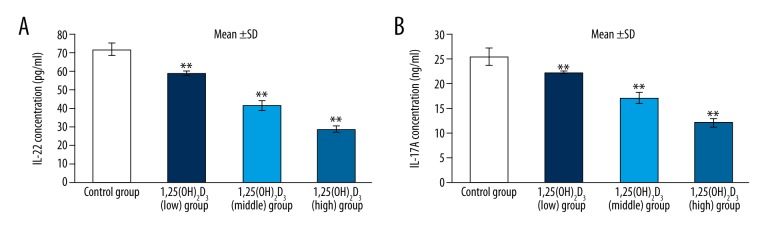
Flow cytometry showed that the proportion of Th17 cells was the most in the control group (62.9%) and reduced in the 1,25(OH)2D3 treatment group and presented as dose dependent. Namely, the proportion of Th17 cells decreased with an increasing 1,25(OH)2D3 concentration (Figure 6). RT-PCR showed that the mRNA levels of IL-17A and IL-22 were significantly decrease in the 1,25(OH)2D3 group compared to those of the control group (p<0.01). RORγt and RORα were specific transcription factors of Th17 cells. Th0 cells differentiated into Th17 cells and expression of RORγt and RORα increased in the control group after inducing differentiation. Compared with the control group, expression of RORγt and RORα were reduced in the 1,25(OH)2D3 treatment group and presented as dose dependent (p<0.01) (Figure 7). The result of cytokines detection in culture supernatant by ELISA (Figure 8) showed that IL-17A and IL-22 concentrations were reduced compared with the control group and they were reduced following 1,25(OH)2D3 concentration increases (p<0.01).
Figure 6.
The intervention of 1,25(OH)2D3 on Th17 cell differentiation.
Figure 7.
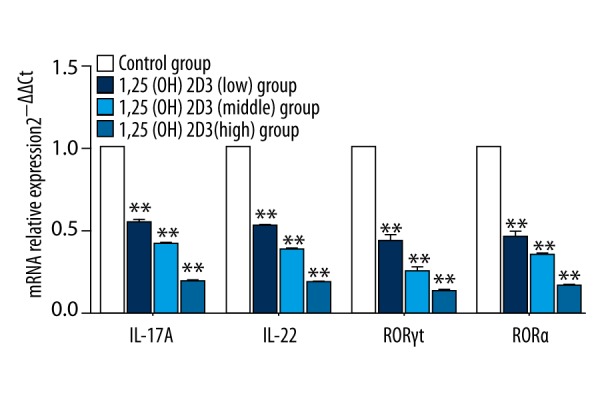
The mRNA expression of specific transcription factors and cytokines of Th17 cells (* means compared with control group, ** p<0.01).
Figure 8.
The concentration of cytokines of IL-17A and IL-22 (* means compared with control group, ** p<0.01).
Discussion
Liver fibrosis is the pathological changes of chronic liver disease. Liver fibrosis has a serious impact on liver function, manifested as a large number of ECM depositions and lack of degradation, and this persistent condition may develop into cirrhosis (liver fibrosis). The degree of liver fibrosis has been positively correlated with the rate of vitamin D deficiency in HIV/HCV superinfection and vitamin D deficient patients [13]. The probability of the occurrence of liver fibrosis has been positively correlated with the rate of vitamin D deficiency in nonalcoholic fatty liver [14]. In addition, some researchers have noted that mutation of 1,25(OH)2D3 metabolism plays an inhibitory effect on liver fibrosis, thus increasing the elasticity of the liver [15]. Abramovitch et al. [16] reported that 1,25(OH)2D3 inhibited the expression of cyclin D1 in mouse HSCs, reducing the proliferation of HSCs, thus affecting reverse transcription and expression of collagen through inhibiting the activation of type I collagen promoter. Studies have also confirmed that 1,25(OH)2D3 can reduce the deposition of ECM and the degree of liver fibrosis in animal models of liver fibrosis. Our study aimed to explore the treatment of 1,25(OH)2D3 in liver fibrosis by constructing models of liver fibrosis in mice. Histopathological analysis (H&E staining and Sirius-red staining) showed that the nucleus boundary was not clear in the model group, and a large number of vacuoles and fibrosis tissue were identified. The recovery of cells in the 1,25(OH)2D3 treatment group was good, with a small amount of fibrosis tissue and vacuoles identified, suggesting 1,25(OH)2D3 could decrease liver fibrosis. T helper cell is a significant immunoregulatory cell, and CD4+T helper cells can be divided into Treg cells, T helper cell 17 (Th17), Th1, and Th2 and other cell subsets. Th17 cells are different from Th1 and Th2 cells, and are novel helper T cells which secreted IL-17 [17]. RORγt (RORC in humans) is a main transcription factor which adjusts initial CD4+cells to differentiate into Th17 cells [18]. Th17 cells and Th1 cells both exist in a number of inflammatory diseases and autoimmune diseases (AID), and regulate the different stages of the immune response by producing specific cytokines [19–21]. Th17 cells and Th1 cells were both involved in the pathogenesis of hepatic fibrosis [22]. Wang et al. [23] found that Th17 and Th1 cells had mutually antagonistic role in differentiation. However, Th17 and Th1 cells can co-exist in a number of inflammatory lesions in autoimmune diseases [24]. Th1 and Th2 cells are important regulatory cells, while mutual inhibiting. Different Th1/Th2 cell types of dominant response play a significant role in the occurrence, development, and prognosis of a number of diseases. This work showed that the proportion of Th1 cells and Th17 cells increased in a mouse model of liver fibrosis, while expression of Th17 and Th1-specific transcription factors (RORγt and T-bet) were increased, respectively. This indicated that ratios of Th17 cell and Th1 cells were positively correlated in liver fibrosis environment. Compared with the control group, the ratio of Th2 cells, content of IL-4, and expression of Th2-specific transcription factor (GATA3) were all reduced. This indicated that Th1 cell and Th2 cells were negatively correlated in the liver fibrosis environment. Imbalance of Th1/Th2 cells may be corrected with 1,25(OH)2D3 in the following ways. 1) Effects activity of NFAT, a T cell nuclear factor, and directly inhibits activity of promoter of IFN-γ (25–26). 2) Inhibits the activity of T-bet (Th1 cell-specific transcription factor) that leads to decreased Th1 cell activity. 3) Increases expression of GATA-3 and c-maf, which are Th2-type transcription factors [27]. 4) Induces immature DC to secret IL-10 to promote the generation of Treg cells and inhibit the activation of Th1 cells by cell-cell-contact [28,29]. 1,25(OH)2D3 can also inhibit the activation of Th17 cells. Khoo et al. [30] found that Candida albicans infection of peripheral blood in the normal population, after giving 1,25(OH)2D3, could significantly decrease the levels of inflammatory cytokines IL-17, TNF-α, IL-6, and IFN-γ. Wahono et al. [31] demonstrated that 1,25(OH)2D3 inhibited dendritic cells maturation and Th17 cells activation in SLE patients and increased Treg cells, but not significantly. Research has shown that after 1,25(OH)2D3 treatment, ratios of Th1 and Th17 cells, cytokine secretion, and mRNA expression of specific transcription factors were reduced, while Th2 cell ratio, IL-4, and mRNA expression of GATA3 increased, indicating that 1,25(OH)2D3 inhibited Th1 and Th17 cells while promoting Th2 cell activation.
Conclusions
Combined with the results that 1,25(OH)2D3 inhibited Th17 cell differentiation in vitro, this study indicated that 1,25(OH)2D3 could effectively regulate the immune response of T cells and participated in the treatment of liver fibrosis and other diseases by regulating the balance of T cells.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by Grant No. 20154Y0207 from Shanghai Municipal Commission of Health and Family Planning Fund
References
- 1.Chen D, Li Y, Dai X, et al. 1,25-Dihydroxyvitamin D3 activates MMP13 gene expression in chondrocytes through p38 MARK pathway. Int J Biol Sci. 2013;9:649–55. doi: 10.7150/ijbs.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramovitch S, Dahan-Bachar L, Sharvit E, et al. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728–37. doi: 10.1136/gut.2010.234666. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Sims GP, Chen XX, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E, Kom T, Oukka M, et al. Induction and effector functions of Th17 cells. Nature. 2008;453:1051–57. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel C, Sartory NA, Zahn N, et al. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper(Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 6.Zehnder D, Quinkler M, Eardley KS, et al. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008;74:1343–53. doi: 10.1038/ki.2008.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez AM, Wongtrakool C, Weleh T, et al. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cells. J Steroid Biochem Mol Biol. 2010;118:l42–50. doi: 10.1016/j.jsbmb.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda M, Yamashita T, Sasaki N, et al. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler Thromb Vasc Biol. 2010;30:2495–503. doi: 10.1161/ATVBAHA.110.215459. [DOI] [PubMed] [Google Scholar]
- 9.Tang J, Zhou R, Luger D, et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol. 2009;182:4624–32. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang JH, Cha HR, Lee DS, et al. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of Th17 cells to protect against experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e12925. doi: 10.1371/journal.pone.0012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee TY, Wang GJ, Chiu JH, et al. Long-term administration of Salvia miltiorrhiza ameliorates carbon tetrachloride-induced hepatic fibrosis in rats. J Pharm Pharmacol. 2003;55:1561–68. doi: 10.1211/0022357022098. [DOI] [PubMed] [Google Scholar]
- 12.Muriel P, Fernández-Martínez E, Pérez-Alvarez V, et al. Thalidomide ameliorates carbon tetrachloride induced cirrhosis in the rat. Eur J Gastroenterol Hepatol. 2003;15:951–57. doi: 10.1097/00042737-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Guzmán-Fulgencio M, García-Álvarez M, Resino S, et al. Vitamin D deficiency is associated with severity of liver disease in HIV/HCV coinfected patients. J Infect. 2014;68:176–84. doi: 10.1016/j.jinf.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Nobili V, Giorgio V, Liccardo D, et al. Vitamin D levels and liver histological alterations in children with nonalcoholic fatty liver disease. Eur J Endocrinol. 2014;170:547–53. doi: 10.1530/EJE-13-0609. [DOI] [PubMed] [Google Scholar]
- 15.Grünhage F, Hochrath K, Lammert F, et al. Common genetic variation in vitamin D metabolism is associated with liver stiffness. Hepatology. 2012;56:1883–91. doi: 10.1002/hep.25830. [DOI] [PubMed] [Google Scholar]
- 16.Abramovitch S, Dahan-Bachar L, Sharvit E, et al. Vitamin D inhibits proliration and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728–37. doi: 10.1136/gut.2010.234666. [DOI] [PubMed] [Google Scholar]
- 17.Cua DJ, Tato CM. Innate IL-17-producing cells: The sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 18.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease inductionaffect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stromnes IM, Cerretti LM, Liggitt D, et al. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–42. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor RA, Prendergast CT, Sabatos CA, et al. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181:3750–54. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Qiu SJ, She WM, et al. Significance of the balance between regulatory T (Treg) and T helper 17 (Th17) cells during hepatitis B virus related liver fibrosis. PLoS One. 2012;7:e39307. doi: 10.1371/journal.pone.0039307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pène J, Chevalier S, Preisser L, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423–30. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Xu J, Niu Y, et al. T-bet and eomesodermin play critical roles in directing T cell differentiation to Th1 versus Th17. J Immunol. 2008;181:8700–10. doi: 10.4049/jimmunol.181.12.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeuchi A, Reddy GS, Kobayashi T, et al. Nucheau factor of activated T cells (NFAT) as a molecular garget for 1, ahpha, 25-dihydroxyvitamin D3 mediated effects. Immounnl. 1998;160:209–18. [PubMed] [Google Scholar]
- 26.Cippitelli M, Santoni A. Vitamin D3: A transcriptional modulator of the interferon-gamma gene. Eur J Immunol. 1998;28:3017–30. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Boonstra A, Barrat FJ, Crain C, et al. 1 alpha, 25-dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 28.Gregori S, Giarratana N, Smiroldo S, et al. A 1alpha 25-dihydroxyvitamin D3 analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–74. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 29.Camara NO, Sebille F, Lechler RI. Human CD4+CD25+ regulatory cells have marked and sustained effects on CD8+ T cell activation. Eur J Immunol. 2003;33:3473–83. doi: 10.1002/eji.200323966. [DOI] [PubMed] [Google Scholar]
- 30.Khoo AL, Chai LY, Koenen HJ, et al. 1,25-dihydroxyvitamin D3 modulates cytokine production induced by Candida albicans: Impact of seasonal variation of immune responses. J Infect Dis. 2011;203:122–30. doi: 10.1093/infdis/jiq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahono CS, Rusmini H, Soelistyoningsih D, et al. Effects of 1,25(OH)2D3 in immune response regulation of systemic lupus erithematosus (Sle) patient with hypovitamin D. Int J Clin Exp Med. 2014;7:22–31. [PMC free article] [PubMed] [Google Scholar]