Abstract
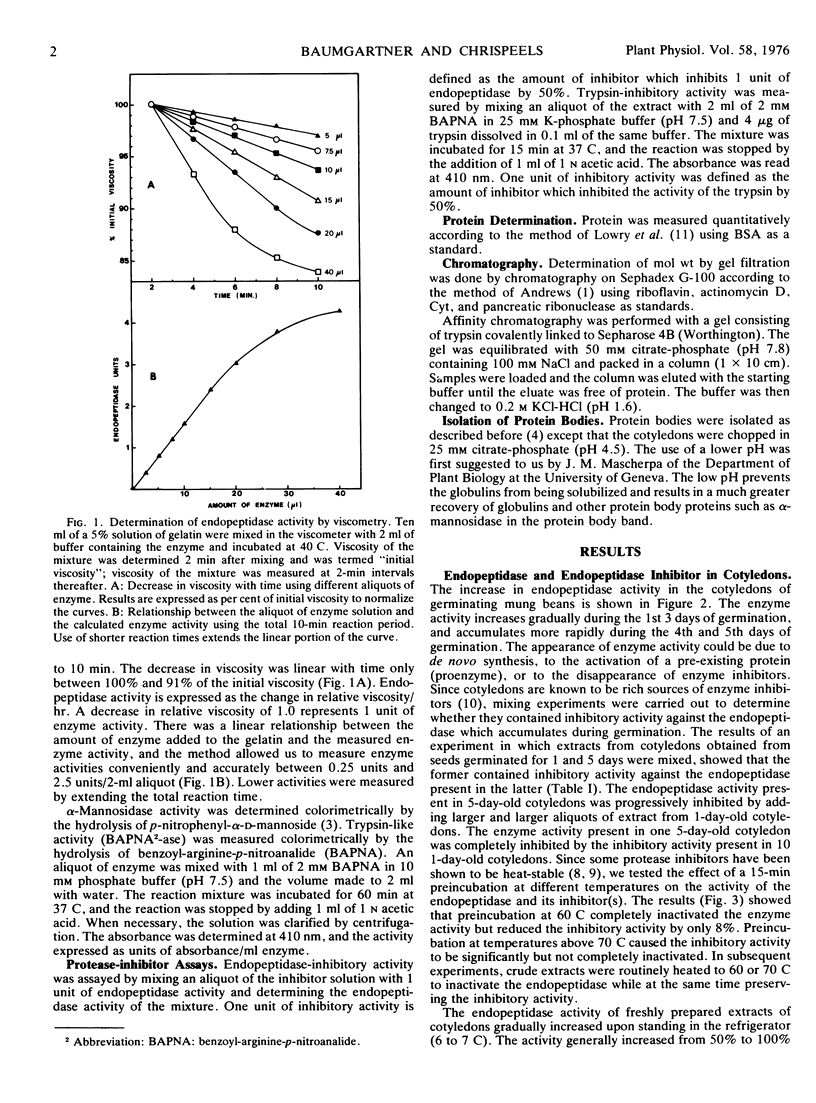
Germination of mung beans (Phaseolus aureus, Roxb.) is accompanied by an increase in the activity of the endopeptidase involved in storage protein metabolism. Enzyme activity in the cotyledons increases 25-fold during the first 5 days of germination. The cotyledons also contain inhibitory activity against the endopeptidase, and this inhibitory activity declines during germination, suggesting that inhibitors may play a role in regulating the activity of the endopeptidase.
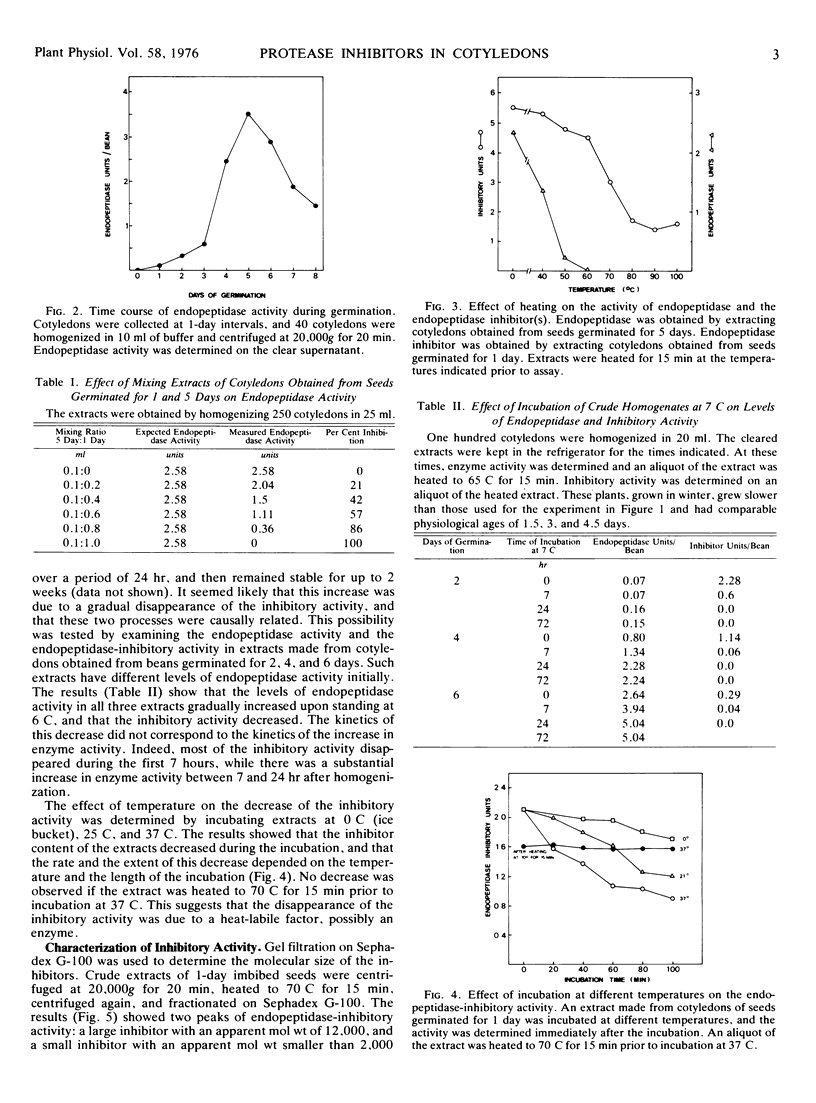
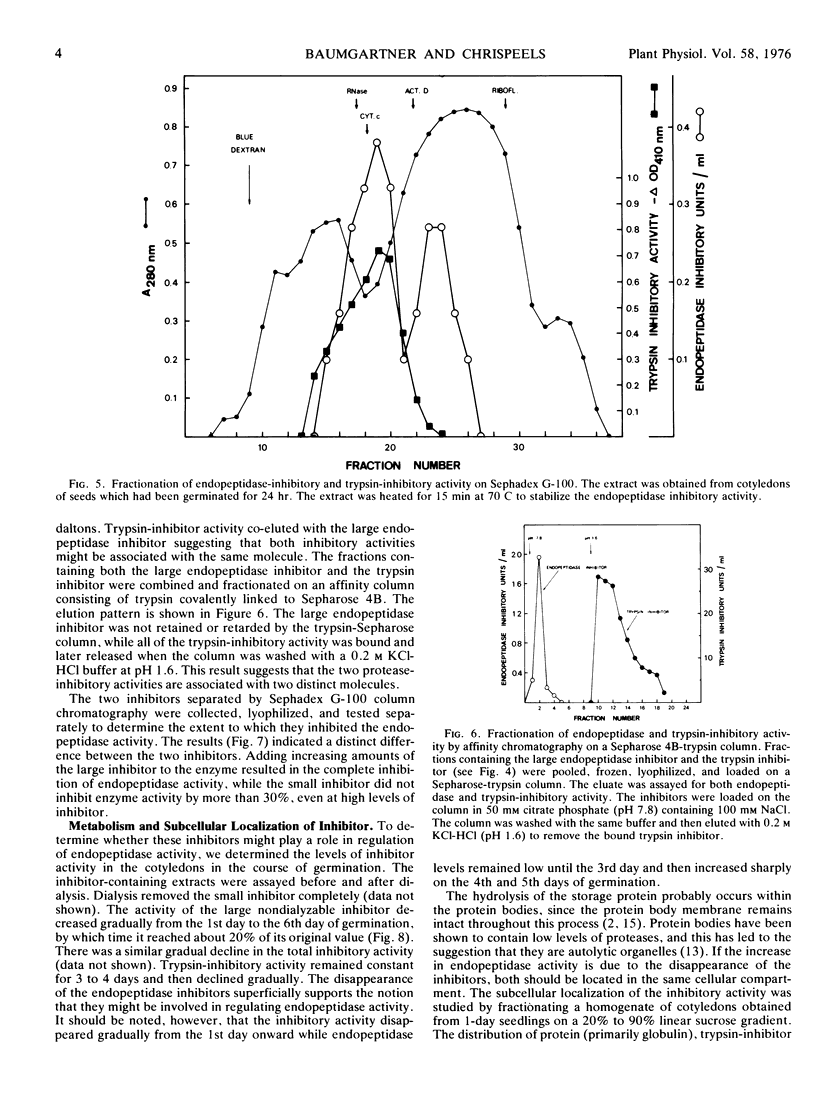
The inhibitory activity against the mung bean endopeptidase is due to the presence of two inhibitors which can be separated by chromatography on Sephadex G-100. The two inhibitors have approximate molecular weights of 12,000 and smaller than 2,000 daltons. The large inhibitor coelutes with trypsin inhibitor on Sephadex G-100, but these two inhibitory activities can be separated by means of a trypsin affinity column.
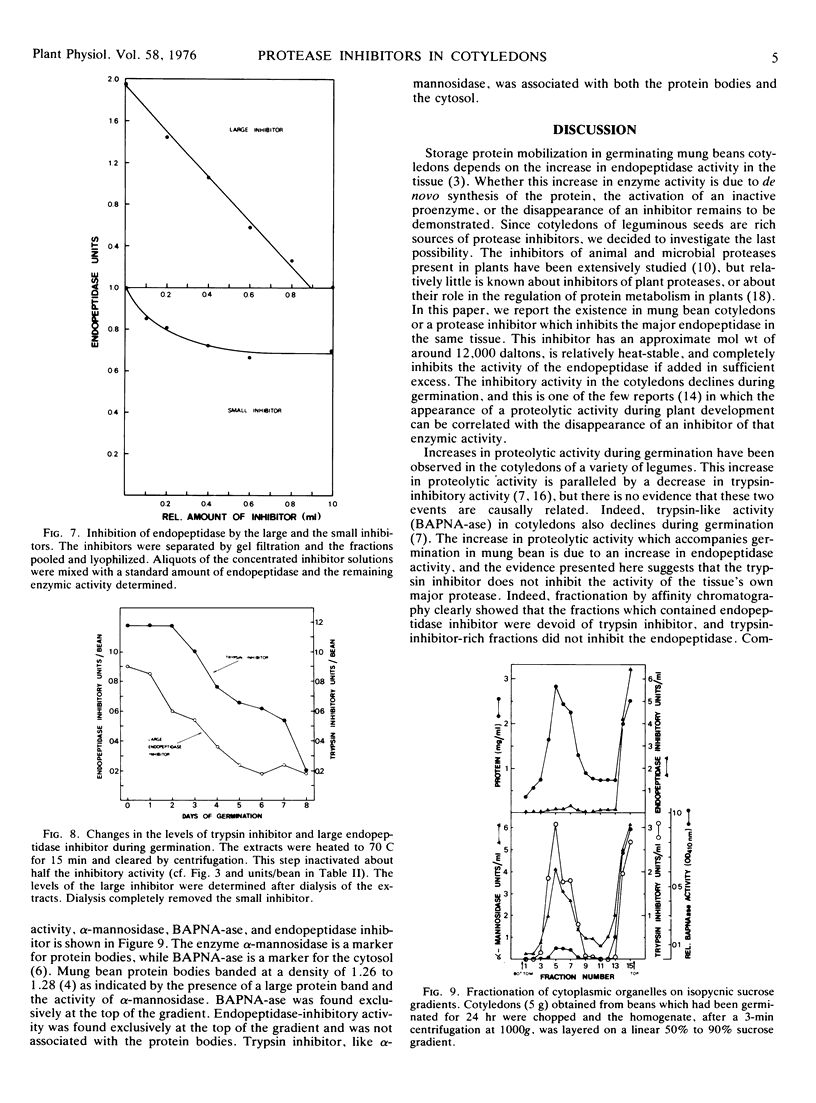
The inhibitory activity disappears slowly from crude extracts incubated at 6 C and more rapidly when the extracts are incubated at 25 C or 37 C. The disappearance of inhibitory activity is accompanied by a rise of the endopeptidase activity, but an examination of the kinetics of these two phenomena suggests that they are not causally related. Fractionation of the cellular organelles on sucrose gradients shows that the inhibitory activity is not associated with the protein bodies, but rather with the cytosol. Our results suggest that the endopeptidase inhibitor(s) does not regulate the increase in endopeptidase activity which accompanies germination or the metabolism of storage protein. We, therefore, postulate that the inhibitor(s) may function in protecting the cytoplasm from accidental rupturing of the protease-containing protein bodies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrispeels M. J., Boulter D. Control of storage protein metabolism in the cotyledons of germinating mung beans: role of endopeptidase. Plant Physiol. 1975 Jun;55(6):1031–1037. doi: 10.1104/pp.55.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. C., Chrispeels M. J. Isolation and Characterization of Glucosamine-containing Storage Glycoproteins from the Cotyledons of Phaseolus aureus. Plant Physiol. 1973 Aug;52(2):98–104. doi: 10.1104/pp.52.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. A., Gunning B. E. Localization of legumin and vicilin in bean cotyledon cells using fluorescent antibodies. Nature. 1970 Oct 3;228(5266):81–82. doi: 10.1038/228081a0. [DOI] [PubMed] [Google Scholar]
- Harris N., Chrispeels M. J. Histochemical and biochemical observations on storage protein metabolism and protein body autolysis in cotyledons of germinating mung beans. Plant Physiol. 1975 Aug;56(2):292–299. doi: 10.1104/pp.56.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Varner J. E., Schidlovsky G. Intracellular Distribution of Proteins in Pea Cotyledons. Plant Physiol. 1963 Mar;38(2):139–144. doi: 10.1104/pp.38.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Association of lysosomal activity with aleurone grains in plant seeds. Arch Biochem Biophys. 1968 Mar 20;124(1):466–471. doi: 10.1016/0003-9861(68)90354-8. [DOI] [PubMed] [Google Scholar]


