Abstract
The prognostic role of vascular endothelial growth factor (VEGF) in cervical cancer is controversial to date. The aim of this study was to evaluate the prognostic value of VEGF and VEGF-C in patients with cervical cancer. Relevant studies were identified by systematic search of the PubMed and Embase database. The primary data of eligible studies was hazard ratio (HR) with 95% confidence interval (95% CI) of survival outcomes, including overall survival (OS), disease-free survival (DFS) and progression-free survival (PFS). Pooled HR (95% CI) was calculated to evaluate the prognostic role of VEGF and VEGF-C in cervical cancer patients. The methodological qualities of the included studies were assessed using REMARK. Fourteen eligible articles including 1306 patients were included in the meta-analysis. The pooled HRs (95% CIs) of VEGF for OS and DFS/PFS were 2.29 [1.27, 4.14] and 2.77 [1.37, 5.62], respectively. The HR (95% CI) of VEGF-C for OS was 3.94 [2.22, 6.99]. This meta-analysis suggested that high expressions of VEGF and VEGF-C were significantly associated with poor survival outcome in cervical cancer patients.
Keywords: VEGF, cervical cancer, prognosis
INTRODUCTION
Cervical cancer is the third most common cancer and the fourth leading cause of cancer-related deaths in females worldwide. Globally, 529,800 new cancer cases were diagnosed and 275,100 cases died from the disease in 2008 [1]. Although cervical cancer incidence rates have declined significantly in developed countries recently, it remains to be one of the most common cancers in women in developing countries [1]. In order to improve survival outcome of cervical cancer, several prognostic biomarkers have been identified. Human-papilloma virus (HPV) related biomarkers have been proven as effective biomarkers in the diagnosis and prevention of cervical cancer [2, 3]. However, the researchers are still screening new markers which are highly associated with cervical cancer progression and prognosis.
Angiogenesis is considered as a very important biological process for primary cancer growth and metastasis. Vascular endothelial growth factor (VEGF) is a significant biomarker causing tumor angiogenesis. It has been demonstrated that the over expression of VEGF is associated with poor survival in various cancer patients, including lung cancer, colorectal cancer, ovarian cancer and some other tumors [4–7]. Meanwhile, several studies have evaluated the prognostic value of VEGF in cervical cancer. However, the results are conflicting or inconclusive. This discrepancy is mostly due to the relatively small sample size, different detecting methods and genuine heterogeneity. In this study, we sought to conduct a meta-analysis to evaluate the prognostic value of high expression of VEGF in patients with cervical cancer.
RESULTS
Eligible studies
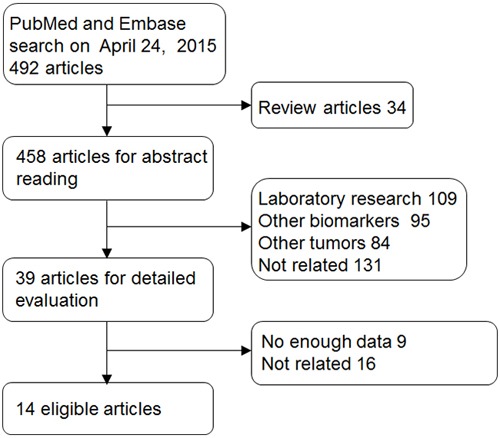
The initial search yielded 492 articles from PubMed and Embase databases after duplicates were removed. The selection process was shown in Figure 1. Handy searches were also conducted for the reference lists of primary studies, review articles, and clinical guidelines. However, no additional eligible studies were retrieved. After screening the titles and abstracts, 453 studies were excluded. Then 25 articles were further excluded after full texts reading according to the criteria. Finally, 14 eligible articles [8–21] including 1306 patients containing survival outcomes were included.
Figure 1. Selection of studies.
Baseline characteristics of studies included in the meta-analysis
These eligible studies were published from 2000 to 2011. The clinical characteristics of patients and other useful information have been listed in Table 1.
Table 1. Characteristics of all identified studies.
| author | year | N | median age | FIGO (I.II/ III.IV) | LNM (yes/no) | initial therapy | histological type | VEGF type | sampling site | sampling time | method | dilution | attitude | survival outcome | cut-off | quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cheng WF | 2000 | 135 | 50 | 135/0 | 28/107 | OP | SCC,Adeno-Ca,Ade, SCNEC | VEGF | tissue | post | IHC | NR | pos | OS,DFS | 112 pg/ml | 18 |
| Gaffney DK | 2003 | 55 | NR | 34/21 | NR | RT | SCC,Adeno-Ca,Ade | VEGF | tissue | post | IHC | 1:20 | pos | OS,DFS | NR | 12 |
| Kang JO | 2004 | 42 | 62 | 36/6 | 20/22 | RT | SCC | VEGF | tissue | post | IHC | 1:10 | pos | OS | 10% | 13 |
| Kim YH | 2010 | 199 | 49 | 199/0 | 31/168 | OP/OP+RT /OP+CCRT |
SCC,Adeno-Ca,Ade | VEGF | tissue | NR | IHC | NR | NR | OS,DFS | NR | 13 |
| Lee IJ | 2002 | 117 | NR | 117/0 | 25/92 | OP/OP+RT/OP +CT/OP+RT+CT |
SCC,Adeno-Ca,Ade | VEGF | tissue | post | IHC | 1:50 | pos | OS,DFS | 50% | 14 |
| Loncaster JA | 2000 | 100 | 49 | 71/29 | NR | RT | SCC,Adeno-Ca,Ade | VEGF | tissue | post | IHC | 1:200 | pos | OS,PFS | NR | 13 |
| Randall LM | 2009 | 173 | 39 | 173/0 | 146/27 | RT/RT+CT | SCC,Adeno-Ca,Ade | VEGF | tissue | post | IHC | NR | neg | OS,PFS | NR | 16 |
| Choi CH | 2008 | 46 | 50 | 29/17 | 11/18 | NAC | SCC,Adeno-Ca | VEGF | tissue | post | IHC | 1:100 | pos | DFS | NR | 12 |
| Fujimoto J | 2004 | 40 | NR | 40/0 | NR | OP | SCC | VEGF-C | tissue | post | ELISA | 1:50 | pos | OS | 300pg/ml | 11 |
| Ma DM | 2011 | 82 | 50 | 82/0 | 36/46 | OP+RT | SCC,Adeno-Ca | VEGF-C | tissue | post | RT-PCR | NR | pos | OS | NR | 12 |
| Ueda M | 2002 | 52 | 50 | 52/0 | 41/11 | OP+RT/OP+CT | SCC,Adeno-Ca,Ade | VEGF-C | tissue | post | IHC | NR | pos | OS | 50% | 12 |
| Hashimoto I | 2001 | 75 | 53 | 61/14 | 61/14 | OP/OP+RT/OP+CT /RT/CCRT |
SCC,Adeno-Ca,Ade | VEGF-C | tissue | pre | IHC | NR | pos | DFS | NR | 14 |
| 53 | 50 | 53/0 | 38/15 | 16/37 | OP/OP+RT/OP+CT | SCC,Adeno-Ca | post | |||||||||
| Bachtiary B | 2002 | 23 | 72 | 18/5 | 8/15 | RT | SCC,Adeno-Ca | VEGF | serum | post | ELISA | NR | pos | PFS | 224pg/ml | 16 |
| Zusterzeel PL | 2009 | 167 | 42 | 151/16 | 23/102 | OP/OP+RT/CCRT/RT/ palliative | SCC,Adeno-Ca,Ade | VEGF | serum | post | ELISA | NR | NR | OS,DFS | 5pg/ml | 16 |
N number of patients; NR, not reference; FIGO International Federation of Gynecology and Obstetrics staging; LNM lymph node metastasis; OP operation; RT radiation therapy; CCRT concurrent chemoradiation; CT chemotherapy; NAC neoadjuvant chemotherapy; SCC, squamous carcinoma; Adeno-Ca, adenosquamous carcinoma; Ade, adenocarcinoma; SCNEC, small cell neuroendocrine carcinomas; G1,2,G3, histological different grade; VEGF, vascular endothelial growth factor; IHC, immunohistochemistry; ELISA, enzyme-linked immunosorbent assay; RT-PCR, reverse transcription-polymerase chain reaction; pos, positive; neg, negative; OS, overall survival; DFS, disease free survival; PFS, progression free survival
Survival outcomes
Seven studies [8, 12, 14–17, 19] including 821 patients were used to evaluate the relation of VEGF expression and overall survival. Figure 2 showed that the pooled HR (95% CI) of these studies for OS was 2.29 [1.27, 4.14] (heterogeneity: I2 = 79%, P<0.001). The pooled HR (95% CI) of 7 studies [8, 10, 12, 15–17, 19] for DFS/PFS was 2.77 [1.37, 5.62] (n=825, I2 = 83%, P<0.001). We also performed a meta-analysis on the prognostic role of VEGF-C in cervical cancer tissue. The combined HR (95% CI) of three studies [11, 18, 20] for OS was 3.94 [2.22, 6.99] (heterogeneity: I2 = 0%, P=0.88). Only one study reported the DFS of VEGF-C in cervical cancer [13], and the HR (95% CI) for DFS in this study was 1.93 [0.96, 3.92]. Two studies reported that high serum VEGF levels in cervical cancer could be used to predict poor survival outcome. The pooled HR (95% CI) of high serum VEGF for DFS/PFS was 2.67 [1.53, 4.64] [9, 11]. Meanwhile, one study reported that the OS was the important endpoint. The HR (95% CI) of serum VEGF [21] for OS was 1.92 [1.01, 3.64]. All above results of meta-analysis were reviewed in Table 2.
Figure 2. Estimated hazard ratios (HRs) and 95% CIs summary.
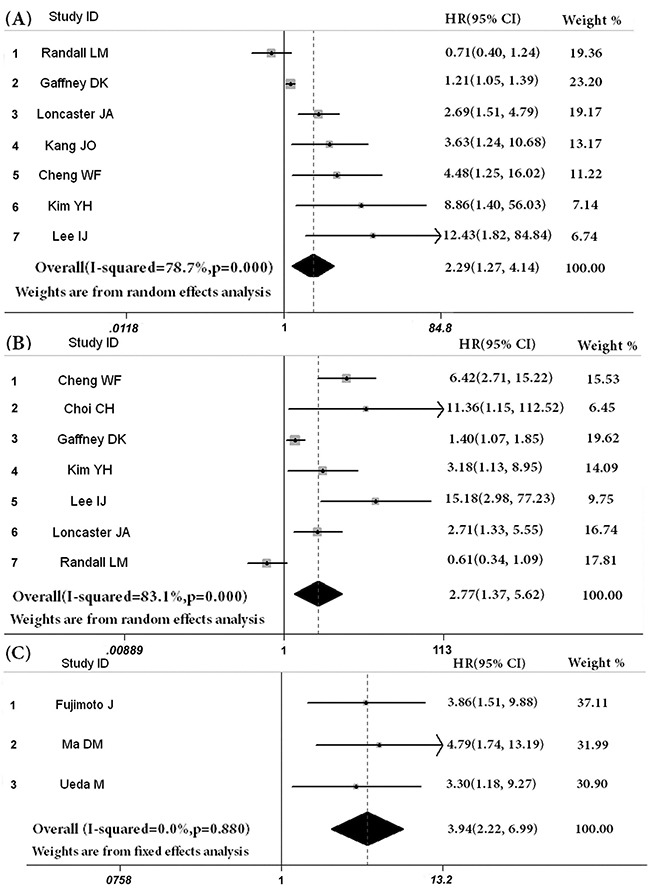
A. overall survival of VEGF expression in cervical cancer, B. disease free survival or progression free survival (DFS/PFS) of VEGF expression in cervical cancer, C. overall survival of VEGF-C expression in cervical cancer.
Table 2. HR (95% Cl) for VEGF and VEGF-C to predict the survival outcome.
| Survival outcome | Study n. | Patient n. | Model | HR (95% Cl) | P value | Heterogeneity (I2,p) | Conclusion | |
|---|---|---|---|---|---|---|---|---|
| VEGF | OS | 7 | 821 | Random | 2.29 [1.27, 4.14] | 0.006 | 79%, <0.0001 | Positive |
| VEGF | DFS/PFS | 7 | 825 | Random | 2.77 [1.37, 5.62] | 0.005 | 83%, <0.00001 | Positive |
| VEGF-C | OS | 3 | 174 | Fixed | 3.94 [2.22, 6.99] | <0.00001 | 0,0.88 | Positive |
| VEGF-C | DFS | 1 | 128 | —— | 1.93 [0.96, 3.92] | 0.07 | —— | —— |
| sVEGF | OS | 1 | 167 | —— | 1.92 [1.01, 3.64] | 0.05 | —— | —— |
| sVEGF | DFS | 2 | 190 | Fixed | 2.67 [1.53, 4.64] | 0.0005 | 34%,0.22 | Positive |
VEGF, vascular endothelial growth factor; sVEGF, serum VEGF; OS, overall survival; DFS, disease free survival; PFS, progression free survival; n, number.
Potential publication bias
Begg's test and funnel plot was used to evaluate the publication bias. No significant publication bias was found in the meta-analysis of VEGF for OS or DFS/PFS. The p-values of Begg's test were 0.051 and 0.176, respectively. We also did the Begg's test in the other groups, and publication bias was not found either.
Assessment of quality
Overall, the global qualitative score of the included studies ranged from 55.0% to 90.0% with a mean score of 68.57%. Concerning the global score, there was no statistically significant difference between the 12 positive [8–12, 14–18, 20, 21] and the 2 negative [13, 19] trials (mean score, 67.5% versus 75%, p =0.531). The difference was not significant in qualitative scores between these fourteen studies with positive and negative conclusion (Mann Whitney test, p=0.312).
DISCUSSION
The prognostic values of VEGF expression have been proven in the recent meta-analyses in gastric cancer, breast cancer, lung cancer and many other cancers. As far as we know, this study was the first meta-analysis revealing the prognostic role of VEGF in cervical cancer. The combined results suggested thathigh expressions of VEGF and VEGF-C were significantly associated with poor survival outcome in patients with cervical cancer. In this meta-analysis, the pooled HRs (95% CI) of VEGF expression for OS and DFS/PFS were 2.29 [1.27, 4.14] and 2.77 [1.37, 5.62], respectively. The intervals of the HRs did not overlap 1, which showed that VEGF could predict the prognosis of cervical cancer patients. As a rule of the thumb, RR>2 suggests that the prognostic factor is useful in clinical practice [22]. The results suggested that high expression of VEGF could be considered as a useful predictive biomarker in cervical cancer. Besides, Sun et al. [23] recently performed a meta-analysis to investigate the association between VEGF expression and lymph node metastasis (LNM) in cervical cancer. They found that VEGF-positive expression was related with higher risk of LNM in cervical cancer, with the odds ratio of 2.87 (95% CI = 1.85-4.44, P < 0.001). Thus, our findings could be partly explained that worse survival might be associated with higher risk of LNM in cervical cancer patients with VEGF-positive expression. Furthermore, Food and Drug Administration (FDA) approved the use of bevacizumab in cervical cancer in 2014 [24] suppressing the biological activity of VEGF, which supports our conclusion from the indirect side.
Although there was significant heterogeneity (I2>50%) between studies in different groups, we have strictly grouped the eligible studies by location, detecting method, cut-off value and VEGF subtype. However, these entire attempts could not eliminate the heterogeneity. Considering these results of subgroup analysis, the heterogeneity of this meta-analysis might be due to the multiply influence of location, detecting method, cut-off value, VEGF subtype and other characteristics.
Three studies [11, 18, 20] were eligible for meta-analysis of prognostic value of VEGF-C in cervical cancer. The combined HR (95% CI) of VEGF-C was 3.94 [2.22, 6.99] in fixed effect model. No significant heterogeneity has been found (p=0.88, I2=0%). Nowadays, VEGF-C has been proven to induce selective hyperplasia of the lymphatic vasculature, which is involved in immune function, inflammation, and tumor metastasis.
This meta-analysis had some limitations. First and foremost, Tierney's method [25] has proved the method is not perfect to calculate the studies based on the Kaplan-Meier survival curve and p-value. We deliberated the data of every article intensively in order to find unreasonable results to rule out. Fortunately, we did not find unreasonable results in these articles. Secondly, we used the software designed by Matthew Sydes and Jayne Tierney [25] to calculate the logHR and SE, which retained only percentile. At the same time, we verified the data again by using Revman, and only minimal bias was observed. Thirdly, although we tried to optimize standardization, some remaining variability like cut-off value in definitions was unavoidable. The cut-off value of VEGF expression could not be in an agreement. Due to lacking of abundant VEGF expression data in global population, it is difficult to set a standard cut-off value.
The publication bias is a major problem in the meta-analysis. Begg's test was chosen in our investigation. We did not find evidence that publication bias might significantly influence our results. However, it should be noted that our meta-analysis could not completely exclude biases. For example, positive results tend to be accepted by journals, while negative results are often rejected or even not submitted, which probably introduced bias.
In conclusion, over expression of VEGF or VEGF-C was associated with poor survival in patients with cervical cancer. These findings suggested that VEGF inhibition therapy could have an important role in cervical cancer, and that VEGF might be routinely examined to predict prognosis in cervical cancer patients. However, more investigations are warranted to further verify our results.
MATERIALS AND METHODS
Search strategy
The electronic databases PubMed and Embase were searched for potential studies on April 24th, 2015. The following key words were used to retrieve titles and abstracts, [cervical] AND ([cancer OR tumor OR carcinoma]) AND ([VEGF] OR [vascular endothelial growth factor]).
Study inclusion/exclusion criteria
Studies were considered eligible if they met all the following inclusion criteria, (i) patients had any type of cervical cancer, (ii) researchers measured the expression of VEGF in tissue or serum, and (iii) studies investigated available data involving the prognostic role of VEGF in cervical cancer patients with survival outcome (OS, DFS/PFS).
Studies were excluded if any following conditions existed, (i) review articles, non-human studies or letters, (ii) duplicated publications, (iii) or lack of adequate information to calculate log hazard ratio (logHR) and SE, following Parmar or Tierney's methods [25, 26]. Abstracts and full texts were reviewed for all searched papers, and reference list were searched for potentially eligible studies according to the above criteria. To avoid duplication data, if more than one study was completed in one particular center, only the biggest one was used.
Data extraction
Two reviewers independently determined study eligibility by reviewing the abstracts and full texts. Disagreements were resolved by consensus. The primary data was survival ratio of VEGF in cervical cancer patients, including hazard ratio (HR) and 95% confidence interval (CI), or the Kaplan–Meier survival curves with log-rank p value. Additional data extracted from the studies included first author, publication year, study size, age, tumor stage, tumor size, lymph node metastasis status, initial therapy, histological classification, methods to detect VEGF, cut-off value, the attitude of conclusion and other clinical characteristics.
Statistical methods
The logHR and SE (logHR) (SE) were used for aggregation of the survival results. However, these statistical variables were not available directly in some studies. We calculated the accessible statistics on the basis of available data with methods developed by Parmar [26] and Tierney [25] instead. The pooled outcomes were presented by Forest plots for estimation of the prognostic value of VEGF expression. Statistical heterogeneity was defined as significant if Q test with p<0.10 or I2≥50%. If there was no significantly statistical heterogeneity (p≥0.10 and I2<50%), a fixed effect model was used for meta-analysis. Otherwise, the random effect models were used in the study [27]. A pooled HR>1 indicated worse outcome for the high expression of VEGF, meanwhile, it would be considered statistically significant if the 95% CI did not overlap 1. The Begg's tests and funnel plots were applied to assess the potential publication bias. If p>0.05, it was considered that there was no significant publication bias [28]. All above calculations were evaluated by using STATA 11.0 (STATA Corporation, College Station, TX).
Assessment of quality
An assessment of study methodology was made according to the previously defined criteria [29]. These principles were used to define 20 individual study characteristics, which were deemed to be the key factors of the studies. For any criterion which was not fulfilled according to the information outlined in the article, one point was deducted from a maximum of 20. Then the scores were calculated by scoring percentile. The qualitative scores were assessed by two independent investigators, and any disagreement was resolved by discussion.
Acknowledgments
We would like to thank Fengtian Wang in figures preparation.
Footnotes
CONFLICTS OF INTEREST
We declare no conflict of interest.
FUNDING
Grant No. 2014K158 from the Administration of Traditional Chinese Medicine of Sichuan; Grant No. 2017SZ0048 from Science and Technology Administration, Sichuan Province; Grant No. 2014SZ0031 from Science and Technology Administration, Sichuan Province; Grant No. 16PJ298 from Health and Family Planning Commission of Sichuan Province; Grant No.2015-RK00-00233-ZF from Science and Technology Administration, Chengdu.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ronco G, Dillner J, Elfstrom KM, Tunesi S, Snijders PJ, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi P, Berkhof J, Peto J, Meijer CJ. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 3.Wang HY, Park S, Kim S, Lee D, Kim G, Kim Y, Park KH, Lee H. Use of hTERT and HPV E6/E7 mRNA RT-qPCR TaqMan assays in combination for diagnosing high-grade cervical lesions and malignant tumors. Am J Clin Pathol. 2015;143:344–51. doi: 10.1309/AJCPF2XGZ2XIQYQX. [DOI] [PubMed] [Google Scholar]
- 4.Gkiozos I, Tsagouli S, Charpidou A, Grapsa D, Kainis E, Gratziou C, Syrigos K. Levels of vascular endothelial growth factor in serum and pleural fluid are independent predictors of survival in advanced non-small cell lung cancer: results of a prospective study. Anticancer Res. 2015;35:1129–37. [PubMed] [Google Scholar]
- 5.Tsai HL, Yang IP, Lin CH, Chai CY, Huang YH, Chen CF, Hou MF, Kuo CH, Juo SH, Wang JY. Predictive value of vascular endothelial growth factor overexpression in early relapse of colorectal cancer patients after curative resection. Int J Colorectal Dis. 2013;28:415–24. doi: 10.1007/s00384-012-1570-z. [DOI] [PubMed] [Google Scholar]
- 6.Cheng D, Liang B, Li Y. Serum vascular endothelial growth factor (VEGF-C) as a diagnostic and prognostic marker in patients with ovarian cancer. PLoS One. 2013;8:e55309. doi: 10.1371/journal.pone.0055309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou X, Wei JC, Fu JH, Wang X, Luo RZ, He JH, Zhang LJ, Lin P, Yang HX. Vascular Endothelial Growth Factor is a Useful Predictor of Postoperative Distant Metastasis and Survival Prognosis in Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2015;22:3666–73. doi: 10.1245/s10434-015-4390-x. [DOI] [PubMed] [Google Scholar]
- 8.Cheng WF, Chen CA, Lee CN, Wei LH, Hsieh FJ, Hsieh CY. Vascular endothelial growth factor and prognosis of cervical carcinoma. Obstet Gynecol. 2000;96:721–6. doi: 10.1016/s0029-7844(00)01025-5. S0029784400010255. [DOI] [PubMed] [Google Scholar]
- 9.Bachtiary B, Selzer E, Knocke TH, Potter R, Obermair A. Serum VEGF levels in patients undergoing primary radiotherapy for cervical cancer: impact on progression-free survival. Cancer Lett. 2002;179:197–203. doi: 10.1016/s0304-3835(01)00872-2. [DOI] [PubMed] [Google Scholar]
- 10.Choi CH, Song SY, Choi JJ, Park YA, Kang H, Kim TJ, Lee JW, Kim BG, Lee JH, Bae DS. Prognostic significance of VEGF expression in patients with bulky cervical carcinoma undergoing neoadjuvant chemotherapy. BMC Cancer. 2008;8:295. doi: 10.1186/1471-2407-8-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto J, Toyoki H, Sato E, Sakaguchi H, Tamaya T. Clinical implication of expression of vascular endothelial growth factor-C in metastatic lymph nodes of uterine cervical cancers. Br J Cancer. 2004;91:466–9. doi: 10.1038/sj.bjc.6601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaffney DK, Haslam D, Tsodikov A, Hammond E, Seaman J, Holden J, Lee RJ, Zempolich K, Dodson M. Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) negatively affect overall survival in carcinoma of the cervix treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:922–8. doi: 10.1016/s0360-3016(03)00209-8. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto I, Kodama J, Seki N, Hongo A, Yoshinouchi M, Okuda H, Kudo T. Vascular endothelial growth factor-C expression and its relationship to pelvic lymph node status in invasive cervical cancer. Br J Cancer. 2001;85:93–7. doi: 10.1054/bjoc.2001.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang JO, Hong SE. The prognostic effect of VEGF expression in squamous cell carcinoma of the cervix treated with radiation therapy alone. J Korean Med Sci. 2004;19:693–7. doi: 10.3346/jkms.2004.19.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YH, Kim MA, Park IA, Park WY, Kim JW, Kim SC, Park NH, Song YS, Kang SB. VEGF polymorphisms in early cervical cancer susceptibility, angiogenesis, and survival. Gynecol Oncol. 2010;119:232–6. doi: 10.1016/j.ygyno.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Lee IJ, Park KR, Lee KK, Song JS, Lee KG, Lee JY, Cha DS, Choi HI, Kim DH, Deung YK. Prognostic value of vascular endothelial growth factor in Stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 2002;54:768–79. doi: 10.1016/s0360-3016(02)02970-x. [DOI] [PubMed] [Google Scholar]
- 17.Loncaster JA, Cooper RA, Logue JP, Davidson SE, Hunter RD, West CM. Vascular endothelial growth factor (VEGF) expression is a prognostic factor for radiotherapy outcome in advanced carcinoma of the cervix. Br J Cancer. 2000;83:620–5. doi: 10.1054/bjoc.2000.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma DM, Xu YP, Zhu L. Expression of vascular endothelial growth factor C correlates with a poor prognosis based on analysis of prognostic factors in patients with cervical carcinomas. J Obstet Gynaecol Res. 2011;37:1519–24. doi: 10.1111/j.1447-0756.2011.01566.x. [DOI] [PubMed] [Google Scholar]
- 19.Randall LM, Monk BJ, Darcy KM, Tian C, Burger RA, Liao SY, Peters WA, Stock RJ, Fruehauf JP. Markers of angiogenesis in high-risk, early-stage cervical cancer: A Gynecologic Oncology Group study. Gynecol Oncol. 2009;112:583–9. doi: 10.1016/j.ygyno.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueda M, Terai Y, Yamashita Y, Kumagai K, Ueki K, Yamaguchi H, Akise D, Hung YC, Ueki M. Correlation between vascular endothelial growth factor-C expression and invasion phenotype in cervical carcinomas. Int J Cancer. 2002;98:335–43. doi: 10.1002/ijc.10193. [DOI] [PubMed] [Google Scholar]
- 21.Zusterzeel PL, Span PN, Dijksterhuis MG, Thomas CM, Sweep FC, Massuger LF. Serum vascular endothelial growth factor: a prognostic factor in cervical cancer. J Cancer Res Clin Oncol. 2009;135:283–90. doi: 10.1007/s00432-008-0442-y. [DOI] [PubMed] [Google Scholar]
- 22.Hayes DF, Isaacs C, Stearns V. Prognostic factors in breast cancer: current and new predictors of metastasis. J Mammary Gland Biol Neoplasia. 2001;6:375–92. doi: 10.1023/a:1014778713034. [DOI] [PubMed] [Google Scholar]
- 23.Sun H, Tang F, Zhou S, Li X, Wang S, Huang K, Jia Y, Tian X, Ma D, Li S. Association between vascular endothelial growth factor expression and lymph node metastasis in cervical cancer: A meta-analysis. J Obstet Gynaecol Res. 2016;42:1310–6. doi: 10.1111/jog.13064. [DOI] [PubMed] [Google Scholar]
- 24.Penson RT, Huang HQ, Wenzel LB, Monk BJ, Stockman S, Long HJ, 3rd, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Method M, Michael H, et al. Bevacizumab for advanced cervical cancer: patient-reported outcomes of a randomised, phase 3 trial (NRG Oncology-Gynecologic Oncology Group protocol 240) Lancet Oncol. 2015;16:301–11. doi: 10.1016/s1470-2045(15)70004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 29.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–4. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]


