Figure 3. Induction of TRPM3/miR-204 and TRPM1/miR-211 upon treatment of melanoma cell lines and metastatic melanoma patients with BRAFi and/or MEKi.
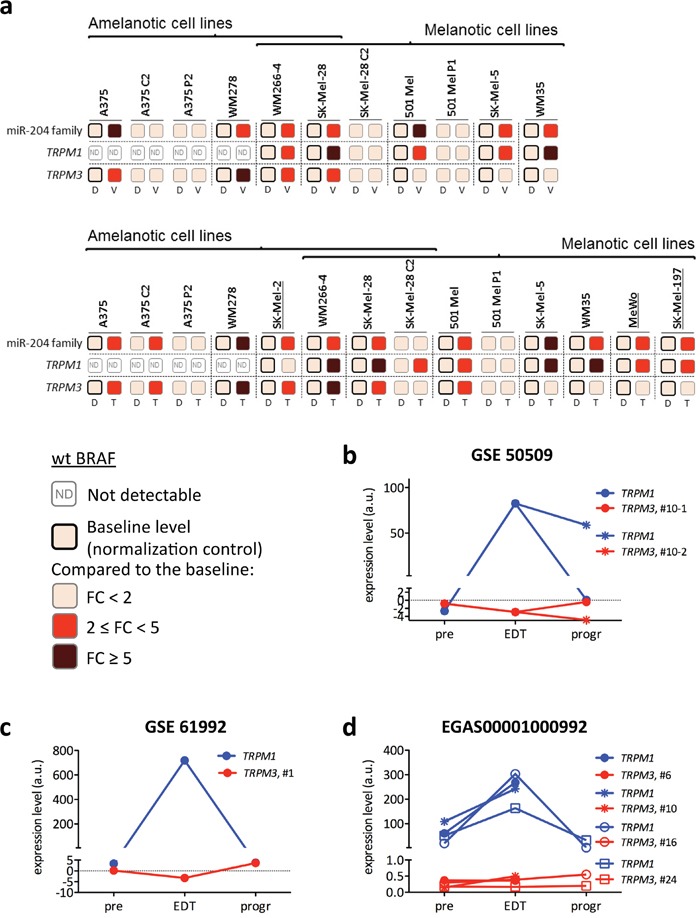
(a) Fold induction of miR-204 family, TRPM1 and TRPM3 upon 48h of 2uM vemurafenib (V, upper) or 1nM trametinib treatment (T, lower) of the indicated BRAFV600E and wt BRAF (underlined) melanoma cell lines. Vemurafenib-resistant clones and populations are used as negative controls (when the drugs cannot function, there is no miRNA induction). D: DMSO. The expression levels of miR-204 family, TRPM1 and TRPM3 in each parental line treated with DMSO is taken as baseline and used as normalization control. Fold changes are therefore represented as increases over the baseline (a darker color means a higher fold change). (b-d) Expression levels of TRPM1 (blue) and TRPM3 (red) in bioptic samples collected from metastatic melanoma patients at 3 time points: before the beginning of the treatment (pre), early on during treatment (EDT) and at progression (progr). (b) In the GSE 50509 dataset, patient #10 was treated with the BRAFi dabrafenib and 2 different tumor sites were analyzed at progression. (c) In the GSE 61992 dataset, patient #1 was treated with the BRAFi dabrafenib and the MEKi trametinib. (d) In the EGAS00001000992 dataset, patients #6, #10 and #16 were treated with the BRAFi dabrafenib and the MEKi trametinib, while patient #24 was treated with the BRAFi vemurafenib.
