Abstract
Aim
To figure out how to correlate the findings on functional MRI and carried out after neoadjuvant CRT of rectal carcinoma with final histology after surgery.
Background
Neoadjuvant CRT is the standard treatment of locally advanced rectal carcinoma. Its use leads to the downstaging of the disease and in 15–42% of patients even to the detection of pCR after TME. The use of functional MRI improves the sensitivity and specificity of pCR detection up to 52–64% and 89–98%, respectively.
Materials and methods
Between January 2013 and June 2016, 67 patients suffering from histologically proven locally advanced rectal cancer underwent neoadjuvant RT or CRT. We selected for further investigation only patients (33 patients) who underwent pelvic staging and restaging using multiparametric imaging on 3T MRI scanner. We compared the findings on functional MRI after neoadjuvant CRT with final histology after surgery.
Results
In 15 patients pathologic staging of primary tumor differed from expected staging assessed according to preoperative MRI. In 5 patients pathologic complete remission was achieved. In none of these 5 patients pCR was predicted using preoperative MRI. Sensitivity and specificity of MRI in predicting pCR were 0% and 96%. Accuracy of MRI in predicting pT and pN was 79% and 74%.
Conclusions
We have verified that the use of neoadjuvant CRT in the treatment of locally advanced rectal carcinoma leads to a possible achievement of pCR. But in our group of patients this was not predictable nor was it with the use of multiparametric 3T MRI.
Keywords: Rectal carcinoma, Neoadjuvant chemoradiotherapy, 3T MRI scanning, Restaging
1. Background
Rectal carcinoma has a relatively high incidence in the Czech Republic with considerable mortality. In 2013, rectal carcinoma was diagnosed in our country in 2239 patients (21.3/100,000), of whom 1075 died (10.23/100,000). Male population is more affected than female population in the ratio 2:1 in these epidemiological indicators.1 Worldwide, a total of 746,000 colorectal carcinomas (isolated data on rectal carcinoma are not available) were diagnosed in men and 614,000 in women in 2012 that makes this tumor the 3rd and 2nd most frequent tumor overall.2
Neoadjuvant chemoradiotherapy (CRT) is the standard treatment of locally advanced rectal carcinoma.3, 4 Its use leads to the downstaging of the disease and in 15–42% of patients even to the detection of pathologic complete remission (pCR) after total mesorectal excision (TME).5 Those patients who achieved pCR after neoadjuvant CRT have longer survival and lower probability of local and distant recurrences.6 Recently, studies have been published which admit (when clinical complete remission (cCR) is achieved) considering the conservative approach in the form of a complete omission of surgery, so-called “watch and wait” strategy (or no immediate surgery), thus avoiding greater postoperative complications.7 However, the possibility of using this approach is heavily dependent on the accurate identification of patients who achieve cCR after CRT. There are several evaluation options – digital rectal examination, endoscopic examination8 or imaging methods, mostly in the form of endorectal ultrasound,9 PET/CT examination10, 11 and magnetic resonance imaging (MRI) or PET/MRI. Positive predictive values of these methods regarding selection of complete responders vary between 17% and 50% and may lead to an underestimation of the findings.12 Traditional MRI examination, however, has very low sensitivity for identifying ypT0 stage (19%), because it cannot distinguish between tumor tissue and fibrotic or edematous tissue after CRT.13 Conversely, the use of functional MRI, such as diffusion weighted imaging (DWI) and dynamic contrast enhanced MRI (DCE MRI), improves the sensitivity and specificity of pCR detection up to 52–64% and 89–98%, respectively.12, 14
2. Aim
In our retrospective study, we tried to figure out how to correlate the findings on MRI using functional display and carried out after neoadjuvant CRT of rectal carcinoma with the final histology after surgery. We also tried to determine the degree of response to neoadjuvant treatment, especially in terms of achieving pCR.
3. Materials and methods
3.1. Patients
Between January 2013 and June 2016, 67 patients suffering from histologically proven locally or locoregionally advanced rectal cancer underwent neoadjuvant radiotherapy or CRT at the Department of Oncology and Radiotherapy, University Hospital in Pilsen. From this cohort, we selected for further investigation only patients who underwent pretreatment pelvic staging and subsequent restaging after completed CRT using multiparametric imaging on 3T MRI scanner, and where complete documentation was available, including surgical protocol and definitive histological examination. 33 patients, who had been treated at our department between June 2013 and June 2016, met these assumptions (see details in Table 1). All patients signed an informed consent.
Table 1.
Patients and disease and treatment characteristics.
| All patients (n = 33) | |
|---|---|
| Sex | |
| Male | 24 (73%) |
| Female | 9 (27%) |
| Age at the time of diagnosis (median, range) | 63 (25–84) |
| Histology | |
| Adenocarcinoma | 31 (94%) |
| Adenosquamous carcinoma | 1 (3%) |
| Squamous cell carcinoma | 1 (3%) |
| Stage | |
| I | 2 (6%) |
| II | 7 (21%) |
| III | 24 (73%) |
| IV | 0 |
| Radiotherapy technique | |
| 3D CRT | 13 (40%) |
| IMRT-SIB | 20 (60%) |
| Concomitant chemotherapy | 29 (88%) |
3D CRT, 3D conformal radiotherapy; IMRT-SIB, intensity modulated radiotherapy – simultaneous integrated boost.
3.2. Neoadjuvant treatment
All the patients underwent planning contrast pelvic CT examination while occupying, also during treatment, the supine position with their arms on the chest. Contouring of target volumes (GTV, CTV, PTV) and organs at risk (bladder, small bowel, femoral heads) was carried out according to the recommendations of ICRU Reports 5015 and 62.16 During radiotherapy planning we used either 3D conformal radiotherapy with the shrinking field technique, or intensity modulated radiotherapy (IMRT) with the technique of simultaneous integrated boost (SIB). We used two planning target volumes: PTV1 – pelvic area and PTV 2 – tumor, mesorectum, presacral area. The fractionation regimens used differed according to the technique: 45 Gy plus 5.4–9 Gy boost in 1.8 Gy per fraction in 3D conformal radiotherapy and 45/50 Gy in 1.8/2 Gy per fraction in IMRT-SIB. In 29 patients (88%) radiotherapy was concurrently combined with chemotherapy (capecitabine 825 mg/m2 orally twice a day throughout treatment) (see details in Table 1).
3.3. MRI examination
All pretreatment (staging) and preoperative (restaging) MRI examinations were carried out at the Department of Imaging Methods at the same hospital using 3T MRI scanner Magnetom Skyra (Siemens, Erlangen, Germany) in the whole-body coil, without any prior rectal preparation. Native and postcontrast examinations after the administration of the paramagnetic contrast medium Dotarem (acidum gadotericum, Guerbet, France) in the amount of 0.2 ml/kg were carried out (see details in Table 2). Restaging MRI examination was standardly carried out 4–6 weeks after the neoadjuvant treatment completion.
Table 2.
Characteristics of the MRI sequences used in this study.
| Type of the sequence | Plane | Contrast agent |
|---|---|---|
| T2 TSE | AX, SAG, COR | No |
| T1 TSE FS | AX | No |
| DWI | AX | No |
| T1 vibe dynam. | AX | Yes |
| T1 TSE FS | AX | Yes |
TSE, turbo spin echo; FS, fat suppression; DWI, diffusion weighted imaging; AX, axial; SAG, sagittal; COR, coronal.
In the beginning, the size of the tumor and its relationship to surrounding structures were evaluated and also the presence and the size of the nodes were assessed. On DWI the diffusion of water molecules in the extracellular space was evaluated. In tumor the tissue cells multiply and enlarge, which causes reduction of extracellular space and limits the diffusion of water molecules. There is a so-called restriction. Diffusion was evaluated either semiquantitatively using maps of apparent diffusion coefficient (ADC) or quantitatively using measurement values. Data obtained from DCE MRI were evaluated using pharmacokinetic analysis (PKA) with the help of software package Tissue 4D (Siemens, Erlangen, Germany). A two-compartment plasma/tissue system was used. The principle of PKA is the evaluation of contrast agent displacement, which moves together with the fluid between intravascular (blood plasma) and extracellular extravascular space in the assessed tissue. There were four basic parameters evaluated: volume transfer constant (Ktrans), rate constant (Kep), elimination constant (Ve) and initial area under the curve (iAUC). Further, the saturation curve was evaluated from the dynamic data and its form was either malignant or benign.
In restaging examination the tumor morphology and the nodal status were assessed, and the complete data set of DWI and PKA was again evaluated as in the pretreatment examination. Obtained values were mutually compared, enabling us to get more information about the behavior and the status of tumor tissue after the treatment.
3.4. Surgery
According to our institutional standards, the definitive surgery was planned 6–8 weeks after the neoadjuvant treatment completion and always after restaging MRI examination. Surgeries were performed in 31 patients (94%) of TME, which was part of low anterior resection in 17 patients (52%) and abdominoperineal amputation of the rectum in 14 patients (42%). In one patient (3%) TEM (transanal endoscopic microsurgery) excision was performed and one patient (3%) was only further observed.
4. Results
4.1. Treatment response according to restaging MRI examination
Based on the evaluation of treatment response after neoadjuvant treatment according to restaging MRI examination, 16 patients (48%) were downstaged in the T category. In 2 patients (6%) the findings were assessed as cCR, i.e. ycT0.
22 (92%) of 24 patients with initial nodal involvement (cN1-2) were downstaged in the N category. And in 21 patients (86%) the nodal involvement status after neoadjuvant treatment was assessed as ycN0. 30 patients (91%) were assessed as ycN0 after neoadjuvant treatment, i.e. including those who were initially assessed as cN0.
4.2. Correlation between the findings on the restaging MRI examination and the surgical findings
32 patients (97%) underwent surgery procedure after neoadjuvant treatment. The median period from neoadjuvant treatment completion to surgery was 8 weeks (the range 3–17 weeks). The median period between restaging MRI and surgery was 4 weeks (the range 1–14 weeks).
Pathologic classifications were as follows: ypT0N0 in 5 patients (16%), ypT1-2N0 in 15 patients (47%), ypT3-4N0 in 5 patients (16%) and any ypT with N+ in 7 patients (22%).
In 15 patients (47%) the final histology (pathologic staging) of primary tumor (T category) differed from expected staging assessed according to preoperative MRI. In 9 patients (28%) pathologic staging in the T category was lower than staging according to MRI (3× ycT3 vs. ypT2, 4× ycT2 vs. ypT0, 1× ycT2 vs. ypT1, 1× ycT1 vs. ypT0), and in 6 patients (19%) pathologic staging was higher (5× ycT2 vs. ypT3, 1× ycT0 vs. ypT2). Pathologic staging of primary tumor remained unchanged in 17 patients (53%) compared with preoperative MRI staging.
With regard to the N category, the final histology differed in only 6 patients (19%) in comparison with preoperative MRI. 1 patient (3%) was postoperatively downstaged (ycN1 vs. ypN0), and 5 patients (16%) were postoperatively upstaged (all ycN0 vs. ypN1). See details in Table 3.
Table 3.
Detailed characteristics related to the changes of the staging and the therapeutic procedures of each patient.
| Patient (no.) | Sex (M/F) | Age | Grade | Pre-CRT stage | Post-CRT stage | Pathological stage | RT technique | CT (Y/N) | Surgical procedure | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 67 | 1 | T3N1 | T3N0 | T2N0 | 3D CRT | Y | LAR | |
| 2 | M | 65 | 1 | T3N1 | T1N0 | T0N0 | 3D CRT | Y | APR | pCR |
| 3 | F | 66 | 1 | T2N2 | T2N0 | T2N0 | IMRT-SIB | Y | LAR | |
| 4 | M | 63 | 2 | T3N0 | T3N0 | T3N0 | 3D CRT | Y | LAR | |
| 5 | M | 63 | 2 | T3N0 | T2N0 | T0N0 | IMRT-SIB | Y | APR | Squamous cell carcinoma; pCR |
| 6 | M | 63 | 2 | T3N2 | T2N0 | T2N0 | 3D CRT | Y | LAR | |
| 7 | M | 63 | X | T4bN1 | T0N0 | T2N0 | IMRT-SIB | Y | LAR | |
| 8 | F | 59 | X | T2N1 | T2N0 | T0NX | IMRT-SIB | Y | TEM | pCR |
| 9 | M | 58 | 2 | T3N1 | T3N0 | T2N0 | 3D CRT | N | LAR | |
| 10 | M | 60 | 2 | T3N2 | T2N0 | T3N1 | 3D CRT | Y | LAR | |
| 11 | F | 59 | 2 | T3N2 | T2N1 | T3N1 | IMRT-SIB | Y | LAR | |
| 12 | M | 59 | 2 | T2N1 | T2N0 | T2N0 | IMRT-SIB | Y | APR | |
| 13 | M | 55 | 3 | T3N1 | T3N1 | T3N1 | 3D CRT | Y | LAR | |
| 14 | M | 56 | 2 | T4bN0 | T2N0 | T2N0 | IMRT-SIB | Y | APR | |
| 15 | F | 51 | 2 | T3N1 | T2N0 | T1N0 | IMRT-SIB | Y | APR | |
| 16 | F | 51 | 1 | T3N1 | T1N0 | T1N0 | IMRT-SIB | Y | LAR | |
| 17 | M | 44 | 2 | T2N1 | T2N0 | T0N0 | IMRT-SIB | Y | LAR | pCR |
| 18 | M | 42 | 3 | T3N1 | T2N0 | T2N0 | IMRT-SIB | Y | LAR | |
| 19 | F | 43 | 3 | T4bN2 | T2N0 | T0N0 | IMRT-SIB | Y | APR | Mucinous adenocarcinoma; pCR |
| 20 | M | 41 | 1 | T2N1 | T2N0 | T3N1 | IMRT-SIB | Y | LAR | |
| 21 | M | 37 | 1 | T3N0 | T3N0 | T3N0 | 3D CRT | Y | LAR | |
| 22 | M | 84 | 3 | T2N0 | T0N0 | – | IMRT-SIB | N | – | Adenosquamous carcinoma; “watch and wait” |
| 23 | M | 79 | 2 | T2N0 | T2N0 | T2N0 | 3D CRT | N | APR | |
| 24 | F | 71 | 3 | T3N2 | T3N0 | T3N1 | 3D CRT | Y | APR | |
| 25 | M | 71 | 2 | T3N1 | T3N0 | T3N1 | IMRT-SIB | Y | APR | |
| 26 | M | 68 | 2 | T3N1 | T3N0 | T3N0 | 3D CRT | Y | APR | |
| 27 | F | 69 | 2 | T3N0 | T2N0 | T3N0 | IMRT-SIB | Y | LAR | |
| 28 | M | 68 | 2 | T3N2 | T2N0 | T2N0 | IMRT-SIB | Y | APR | |
| 29 | M | 68 | 2 | T3N0 | T3N0 | T2N0 | 3D CRT | Y | APR | |
| 30 | M | 52 | 2 | T4aN2 | T3N0 | T3N1 | IMRT-SIB | Y | LAR | |
| 31 | F | 25 | 3 | T3N0 | T2N0 | T3N0 | IMRT-SIB | Y | APR | |
| 32 | M | 80 | 1 | T2N2 | T2N0 | T2N0 | IMRT-SIB | N | APR | |
| 33 | M | 70 | 1 | T2N1 | T2N1 | T2N0 | 3D CRT | Y | LAR |
Pre-CRT, pre-chemoradiotherapy; post-CRT, post-chemoradiotherapy; IMRT-SIB, intensity modulated radiotherapy-simultaneous integrated boost; 3D CRT, three dimensional conformal radiotherapy; LAR, low anterior resection; APR, abdominoperineal resection; pCR, pathological complete regression.
4.3. Clinical and pathologic complete remission
In 5 patients (16%) the pathologic complete remission was achieved. In none of these 5 patients pCR was predicted using preoperative MRI (4× ycT2N0, 1× ycT1N0). The median period between restaging MRI and surgery in this group of patients with pCR was 4 weeks (the range 2–8 weeks).
Only in 2 patients (6%) the treatment response was assessed as cCR (ycT0N0) according to preoperative MRI. In one case the response was underestimated, because the postoperative pathologic staging was ypT2N0, and in the latter case the patient was further observed on a “watch and wait” basis. See details in Table 3.
Sensitivity and specificity of MRI examination in predicting pT0, pN0 and pCR and its diagnostic accuracy are separately shown in Table 4.
Table 4.
Sensitivity and specificity of MRI examination in predicting pT0, pN0 and pCR and its diagnostic accuracy.
| Pathologic findings | Sensitivity | Specificity |
|---|---|---|
| pT0 | 0% | 96% |
| pN0 | 96% | 29% |
| pCR | 0% | 96% |
| Diagnostic accuracy of preoperative MRI | ||
| pT stage | 79% | |
| pN stage | 74% | |
pCR, pathologic complete remission.
5. Discussion
Advances in imaging methods, surgery, radiation and clinical oncology in recent decades have brought significant improvements in treatment outcomes in patients with rectal cancer. The choice of treatment strategy of localized rectal carcinoma is based on clinical examination, together with endoscopic examination, MRI and endorectal ultrasound, and currently follows the recommendations of ESMO (European Society for Medical Oncology) and NCCN (National Comprehensive Cancer Network).17, 18, 19 Neoadjuvant CRT is the standard treatment of locally advanced rectal carcinomas, especially based on the results of the work of the German Rectal Cancer Study Group, first published in 20043 and after prolonged follow-up also in 2012.4 In this study, the group with preoperative CRT was found to show a significant decrease of local recurrences (6% vs. 13%; p = 0.006), higher number of sphincter sparing surgery for low seated tumors (39% vs. 19%; p = 0.004) and a lower toxicity profile.3, 4 Accepted standards for neoadjuvant treatments allow the use of accelerated schemes (5 fractions) with immediate surgery or normofractionated radiotherapy combined with fluoropyrimidine based chemotherapy and followed with surgery after 6–8 weeks.19, 20 Normofractionated neoadjuvant CRT has higher number of pCR rates and a lower number of positive circumferential margins than accelerated radiotherapy.21 For sensitization either intravenous 5-fluorouracil or its oral pro-drug capecitabine may be used with the same effect in terms of pCR, early local recurrences or DFS.22
The use of neoadjuvant CRT leads to the downstaging of the disease and in 15–42% of patients even to pCR after TME. Generally, any response to treatment is a kind of an early indicator that correlates with the results of treatment.23 Patients who achieved pCR after CRT have the longest survival and the lowest likelihood of local and distant recurrences.5, 6 In our retrospective study, the downstaging evaluated by MRI was achieved in 16/33 patients (48%) in the primary tumor area and in 22/24 patients (92%) in the area of nodal involvement. After final surgery with TME, pCR was described in 5 patients (16%), which is an amount that is consistent with the literature. One patient was even initially classified as cT4bN2 and after neoadjuvant treatment as ycT2N0. The remaining four cases were initially classified as cT3N1, cT3N0 and 2× cT2N1.
The fact that patients who achieved pCR have a better prognosis than those with residual finding leads logically to even greater efforts to achieve pCR. One option is to extend the interval between neoadjuvant therapy and surgery. Several studies have clearly shown that patients who underwent surgery more than 7–8 weeks after completion of neoadjuvant therapy achieved more pCRs than those who had their surgery earlier.24, 25, 26, 27 The most appropriate timing for surgery should be balanced between the period of the maximal response of the tumor, the time of the beginning of cancer cells repopulation and the time when tissue changes occur (swelling, fibrotic changes) affecting postoperative morbidity, i.e. in the range of 7–10 weeks.28 In our group the median time from the completion of neoadjuvant therapy to surgery was 8 weeks, most patients were operated in the range of 6–12 weeks. One patient underwent surgery after 17 weeks from completion of neoadjuvant therapy because of heart failure just prior to surgery.
Good long-term results of treatment can nevertheless result in significant symptoms of postoperative morbidity such as prolonged urinary and sexual problems, fecal incontinence with frequent need of temporary or permanent stoma. In these cases, the strategy of “watch and wait” can be an alternative that can help avoid more postoperative complications.7 In 2006 the study by Brazilian authors Habr-Gama et al.30 was published describing the cohort of 361 patients with rectal carcinoma cT2-4N0-1 achieving sustained cCR in 99 patients (27%) after neoadjuvant CRT. Sustained cCR was defined as the absence of residual ulceration, stenosis or tumor mass in the rectum evaluated by DRE or proctoscopy more than 12 months. Only in 5 patients (5%) endorectal recurrence occurred after 18, 43, 56, 64 and 79 months of follow-up, whereas 7 patients (7%) experienced distant recurrence, 5-year disease-free survival (DFS) was 85% and overall survival (OS) was 93%. In 2011, Maas et al.31 published a retrospective study, which included 192 patients with rectal carcinoma cT1-3N0-2, who were treated with neoadjuvant CRT. Of this group, 21 patients (11%) achieved cCR and were followed up on a “watch and wait” basis. Criteria of cCR were extended to evaluation by MRI. At a median follow-up of 25 months endorectal recurrence appeared only in one patient who subsequently underwent TEM excision of this recurrence. Control group for comparison consisted of 20 patients achieving pCR after TME. 2-year OS and DFS in the “watch and wait” group were 100% and 89%, respectively; and in the control group, 91% and 93%, respectively. These studies show that proper evaluation of cCR and tight follow-up enable careful monitoring of patients, who can avoid immediate surgery and still have good therapeutic results. This conservative approach is very useful especially for elderly patients and for those with severe comorbidities. But this approach requires cCR to be precisely identified by the clinical and radiological signs.
The current effort is correct and accurate assessment of cCR after neoadjuvant treatment. There are several possibilities of assessment. Firstly, there is DRE, in which cCR is assessed as the absence of any irregularity of the rectal wall and the surface must be regular and smooth. It is also very important endoscopic evaluation in which no residual tumor mass, ulceration or stenosis should be present.8 Regarding imaging methods, high frequency 3D endorectal ultrasound can also identify residual tumor and complete response with a high degree of reliability, although the findings should be evaluated comprehensively with other imaging methods and endoscopy.9 On the other hand, the position of PET/CT in the evaluation of complete remission is not yet entirely clear. Some studies have reported relatively good accuracy, e.g. Perez et al.10 indicate the accuracy of 85%, while Guillem et al.11 in their prospective study describe that only 54% of patients with pCR were classified as cCR on preoperative PET scan and only 19% on CT scan.
MRI examination is important for the staging of patients with rectal cancer. Sensitivity and specificity for evaluation of T staging is 81–92% and 68–80%, respectively.32 MRI shows 92% specificity in predicting negative circumferential margins, which is the only preoperative parameter significant for overall survival, disease-free survival and local recurrence.33 For evaluation of anatomical proportions mainly T2-weighted images without fat signal suppression in three dimensions are used, which allow a good resolution of each structure, especially the external boundary wall of the rectum and the relationship of the tumor to the surrounding structures. MRI is the only method able to assess the relationship of the tumor mass to mesorectal fascia, whose intactness is an indicator of the possibility to perform R0 surgery. Each node measuring 5 mm or more in its short axis is considered to be a pathologically enlarged node in the perirectal fat tissue. However, studies show that the size of the nodes is just tentative and it cannot clearly indicate the presence of metastases. Micrometastases may occur in very small nodes, while nodes larger than 5 mm may be without the evidence of the tumor. Among the parameters indicative of lymph node involvement are irregular edges, and mixed signal intensity, but not the size criteria.34 MRI can assess the status of nodes in the entire mesorectum and in the entire pelvis, unlike endoultrasound that can assess only perirectal nodes.35 Regarding nodal staging, sensitivity of MRI is 77% and specificity 60%.13 Morphological MRI has, in the process of restaging after neoadjuvant treatment, low sensitivity and specificity for the identification of pT0 finding (19% and 94%, respectively), which is due to the limited ability to distinguish between peritumoral fibrosis, desmoplastic reaction, edema or tumor residue after CRT.13, 36 The tumor cannot change the size after treatment, the change occurs only in its structure. The negative predictive value of MRI in the evaluation of residual nodal involvement, necessary to consider the possibility of the “watch and wait” approach, is 81–100%.37
Accuracy of the evaluation of residual tumor and nodal involvement using morphological MRI was explored for example by Kulkarni et al.38 On a group of 80 patients with locoregionally advanced rectal carcinoma, who underwent neoadjuvant CRT followed by surgery, they tried to determine the accuracy of the evaluation of categories yT and yN. Thus, they compared the identified stages ycT and ycN versus ypT and ypN. Accuracy of the evaluation of the T category was 43% (34/80), in 38% of patients were found to be overstaged and 20% were found to be understaged. Accuracy of the evaluation of the N category was 78% (62/80), 4% of patients were found to be overstaged, while 19% were found to be understaged. In a similar study, Suppiah et al.39 described a group of 49 patients with locoregionally advanced rectal carcinoma with the accuracy of the evaluation of the T category being 45% (22/49). Overstaging on MRI was found in 33% of patients and understaging in 22%. Accuracy of the evaluation of the N category was 71% (35/49) with 82% sensitivity and 68% specificity. Positive predictive value was 43% and negative predictive value was 93%. Pathologic CR was reported in 10% (5/49), wherein only one of these patients was reported as ycCR.
Lately, there are efforts to obtain further information about the nature and behavior of the tumor. This is facilitated by the inclusion of new investigative modalities, such as DWI and DCE MRI with PKA (Fig. 1, Fig. 2).40 Using the functional MRI has improved sensitivity and specificity in the detection of pCR – DWI increased accuracy in the detection of viable tumor cells from 64–76% to 86–90%.14 One of the largest studies on this topic was a multicenter study published in 2011 by Lambregts et al.12 The authors describe a group of 120 patients who received neoadjuvant CRT for locally advanced rectal carcinoma, improving the accuracy of detection of pCR by adding DWI to T2-weighted sequences. Sensitivity of DWI for selection of complete responders was 52–64% (compared to 0–40% for only T2-weighted images), and specificity was in both cases 89–98%. Still, suboptimal sensitivity values may be due to the fact, that if the signal in the rectal wall is not fully suppressed, which happens frequently when the rectal wall is collapsed, high signal at the site of the original tumor may be erroneously interpreted as residual tumor.
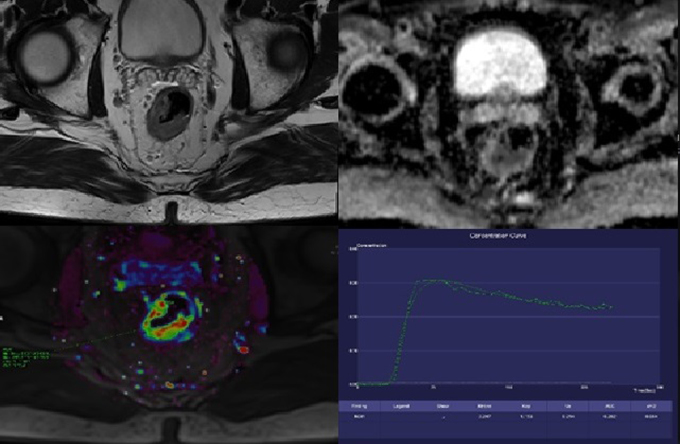
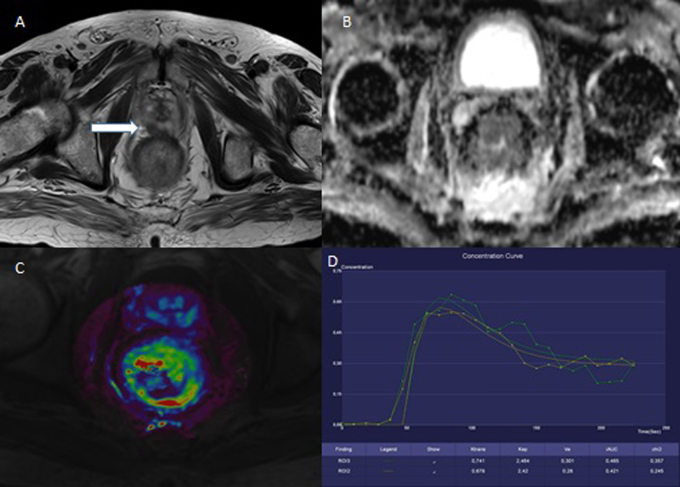
Fig. 1.
71-year-old man with the carcinoma of the middle rectum. The baseline examination. A – T2W, axial plane; the tumor is on the right and posterior wall and invades the serosa, T3aN2M0. B – ADC map; the restriction of diffusion is clearly seen in the tumor site. C – the color map of Ktrans parameter; the increased value (red and yellow color) is seen in the tumor site. D – the saturation curve; the malignant form.
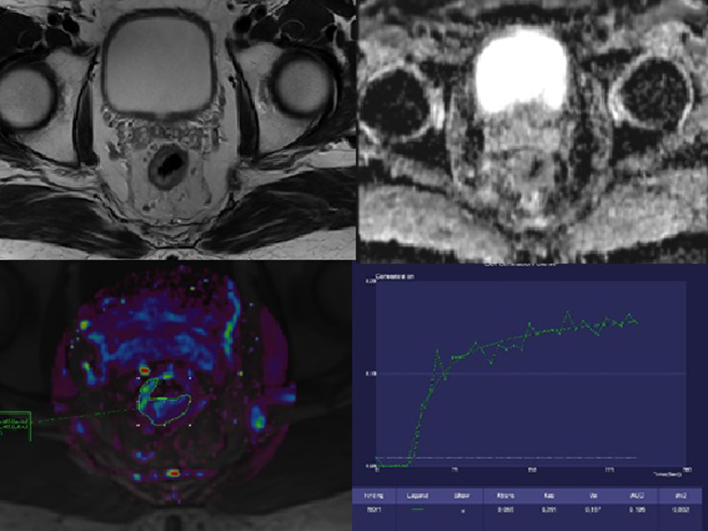
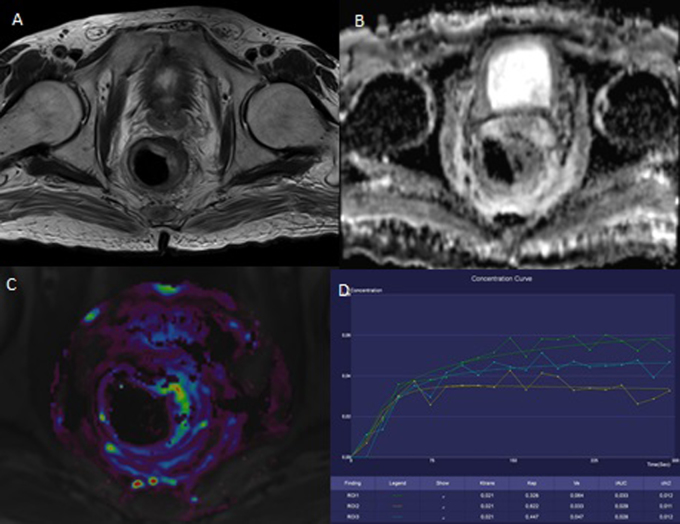
Fig. 2.
The same patient as on Fig. 1. There are signs of good treatment response. A – T2W, axial plane; the narrowing of the rectal wall. B – ADC map; the disappearance of the restriction of diffusion. C – the color map of Ktrans parameter; the decreased value is seen in the tumor site (red and yellow colors disappeared). D – the saturation curve; the benign form.
In our cohort of patients the accuracy of evaluation of residual tumor and nodal involvement was 53% (17/32) and 84% (27/32), respectively. Among inaccurately assessed patients, the overestimation of the T category on MRI occurred in 5 cases after treatment (4× ycT2, 1× ycT1) in those patients who had no evidence of the presence of viable tumor tissue in the final histology (ypT0). Decision on complete response to treatment is, despite a number of additional information that we get using multimodal MRI, very difficult. All overestimated patients had one of the complicating findings. In one patient, there was poor DWI on restaging examination, data were not evaluable. 3 patients had inflammatory changes of the base of the tumor after irradiation. Inflammation in the tissue causes, by the multiplication of blood vessels and by the increasing of wall permeability, the increase of transfer of fluid between compartments and thus causes the changes in PKA maps that mimic the tumor. One patient had mucinous type of carcinoma. In this type of carcinoma, there is an increased amount of mucin in the tumor tissue, which imitates the fat on MRI and, hence, it is hyperintensive on T2- and T1-weighted images (Fig. 3, Fig. 4). On DWI, increased diffusion of water molecules occurs in mucin deposits and therefore restriction of diffusion, typical for high-cell tumor tissue, is missing. The move of contrast agent between compartments is not evaluable and PKA maps show outages in the site of mucin deposits. Changes after treatment in the mucinous tumor can be evaluated only from standard images, the use of other data from multimodal MRI has no sense for the aforementioned reasons. In one case there was an underestimation of the T stage, on MRI a complete response to treatment (ycT0) was reported. The final histology revealed the presence of viable tumor cells only in tunica muscularis propria, in the rest of the specimen there was a good response to treatment, classified as ypT2 (Fig. 5, Fig. 6). Given the distinctiveness of MRI, currently it is impossible to distinguish a small tumor residue, especially in the surface wall portion, without involvement of the mucosa.
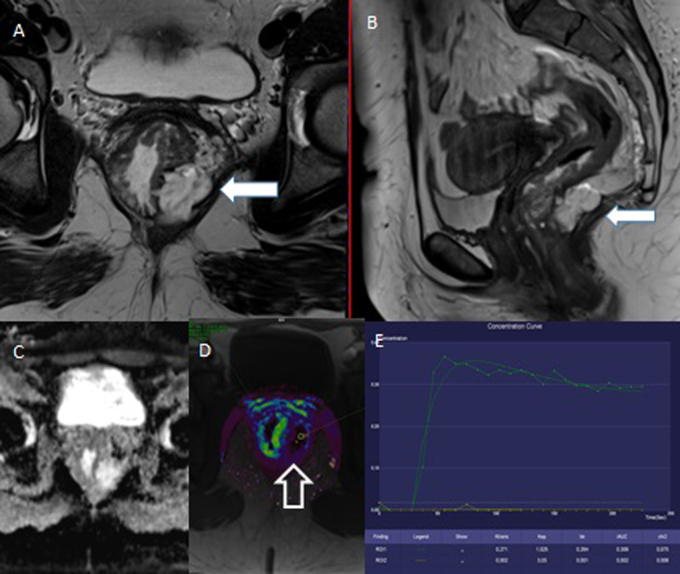
Fig. 3.
45-year-old woman with the carcinoma of lower and middle rectum. The baseline examination. A, B – T2W, axial and sagittal plane; the circularly growing tumor infiltrating the rectovaginal septum and musculus levator ani with mucin deposits (white arrow) prominent dorsally left, T4bN2. C – the tumor does not show typical restriction of diffusion, in mucinous component is increased diffusion. D – only little increasing values of Ktrans parameter, in mucin area they are unmeasurable (transparent arrow). E – the saturation curve does not have typoval malignant shape, in mucin area there is a poor saturation (mucin – yellow color).
Fig. 4.
The same patient as on Fig. 3. The posttreatment examination. A, B – minimal change of the size, mucin deposits are unchanged. C – no restriction of diffusion as on baseline examination. D – unchanged finding on color maps of Ktrans parameter. E – the saturation curves with no change, it does not have malignant shape (mucin – yellow color; muscle – blue color). (Nevertheless histologically pT0N0.)
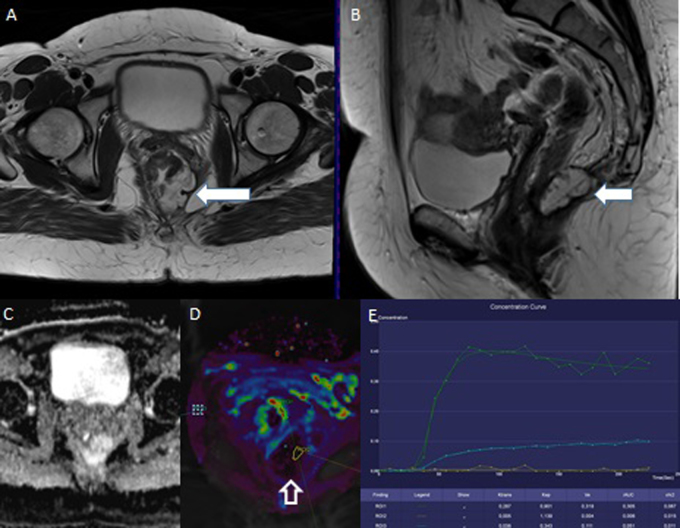
Fig. 5.
62-year-old man with the carcinoma of the lower rectum. The baseline examination. A – T2W, axial plane; the circularly growing tumor infiltrating the prostate (white arrow), T4bN1. B – the restriction of diffusion on ADC map. C – the increased values of Ktrans parameter are shown in red. D – the saturation curve has malignant shape.
Fig. 6.
The same patient as on Fig. 5. The posttreatment examination. A – the reducing tumor size, no signs of serosa penetration. B – the disappearance of the restriction of diffusion. C – the decreasing values of Ktrans parameter. D – the saturation curve has benign shape. (Uncaught little bearing residue of viable tissue in the muscularis propria layer.)
As part of the assessment of our patients using multiparametric MRI. We used, as we already mentioned, PKA, which seems to be a very promising method, but so far little researched. Its evaluation and comparison of results before and after treatment provides additional information about the internal structure of the treated tumor and thus it could distinguish patients who responded well to treatment from those with a large tumor residue. And its potential in terms of prediction of significant therapeutic response is so far inconclusive. In several studies a high value of the Ktrans parameter on pretreatment MRI is associated with a significantly higher chance of achieving downstaging and pCR.41, 42 Conversely, in the study by Kim et al.43 this assumption was not confirmed, Ktrans value on the pretreatment MRI did not differ in patients with different therapeutic responses. Korcakova et al.44 assessed in their study the treatment response of rectal carcinoma to neoadjuvant treatment in 22 patients using multiparametric MRI. Patients were divided into two groups – responders (good response to treatment, i.e. T stage decreased between baseline MRI and histologically identified tumor stage after surgery) and nonresponders (poor response to treatment). Comparing pharmacokinetic parameters and ADC values from the baseline MRI within the group of responders and nonresponders, statistically significantly lower value was found only for the parameter Ve in the group of responders; the differences in all other parameters were not statistically significant. Comparing the results of the baseline examination with examination after treatment separately in the group of responders and nonresponders, in the group of responders statistically significant changes were found in values ADC, Ktrans, Kep and iAUC. In the group of nonresponders, a statistically significant difference was found only in the parameter Ve. In other words, currently, it is not possible to determine from the baseline examination the patients who will respond well to neoadjuvant treatment. But it seems that, based on the comparison of results of PKA and ADC values from baseline and posttreatment MRI, it is possible to determine, whether the patient responded well to treatment and it is expedient to consider less radical surgery or the “watch and wait” approach.
6. Conclusions
We have verified that the use of neoadjuvant chemoradiotherapy in the treatment of locally and locoregionally advanced rectal carcinoma leads to a possible achievement of pathologic complete remission. In our group of patients this was not predictable nor was it with the use of multiparametric 3T MRI. On the contrary, two expected complete responses according to MRI were not subsequently histologically confirmed in one case, and in the latter case the surgery was not performed. Currently, we cannot consider restaging multiparametric MRI examination after neoadjuvant chemoradiotherapy as sensitive enough to predict pathologic complete remission. Therefore, it is advisable to consider a conservative approach in on a “watch and wait” basis only in patients of advanced age or in patients with high surgical risk, who achieved the clinical complete response according to MRI. Multidisciplinary approach in the treatment of rectal carcinoma requires close collaboration of surgeons, medical and radiation oncologists, radiologists and pathologists.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Dušek L., Mužík J., Kubásek M., Koptíková J., Žaloudík J., Vyzula R. Masarykova univerzita; 2005. Epidemiologie zhoubných nádorů v České republice [online] [cit. 2016-8-03]. Dostupný z: http://www.svod.cz. Verze 7.0 [2007], ISSN 1802-8861. [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Ervik M. International Agency for Research on Cancer; Lyon, France: 2013. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet] Available from: http://globocan.iarc.fr [accessed on 03.08.16] [Google Scholar]
- 3.Sauer R., Becker H., Hohenberger W. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(October (17)):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Sauer R., Liersch T., Merkel S. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(June (16)):1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 5.Sanghera P., Wong D.W., McConkey C.C., Geh J.I., Hartley A. Chemoradiotherapy for rectal cancer: an updated analysis of factors affecting pathological response. Clin Oncol (R Coll Radiol) 2008;20(March (2)):176–183. doi: 10.1016/j.clon.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Maas M., Nelemans P.J., Valentini V. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(September (9)):835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 7.Habr-Gama A., Perez R.O., Nadalin W. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(October (4)):711–717. doi: 10.1097/01.sla.0000141194.27992.32. [discussion 717–8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habr-Gama A., Perez R.O., Wynn G., Marks J., Kessler H., Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53(December (12)):1692–1698. doi: 10.1007/DCR.0b013e3181f42b89. [DOI] [PubMed] [Google Scholar]
- 9.Murad-Regadas S.M., Regadas F.S., Rodrigues L.V. Role of three-dimensional anorectal ultrasonography in the assessment of rectal cancer after neoadjuvant radiochemotherapy: preliminary results. Surg Endosc. 2009;23(June (6)):1286–1291. doi: 10.1007/s00464-008-0150-3. [DOI] [PubMed] [Google Scholar]
- 10.Perez R.O., Habr-Gama A., Gama-Rodrigues J. Accuracy of positron emission tomography/computed tomography and clinical assessment in the detection of complete rectal tumor regression after neoadjuvant chemoradiation: long-term results of a prospective trial (National Clinical Trial 00254683) Cancer. 2012;118(July (14)):3501–3511. doi: 10.1002/cncr.26644. [DOI] [PubMed] [Google Scholar]
- 11.Guillem J.G., Ruby J.A., Leibold T. Neither FDG-PET nor CT can distinguish between a pathological complete response and an incomplete response after neoadjuvant chemoradiation in locally advanced rectal cancer: a prospective study. Ann Surg. 2013;258(August (2)):289–295. doi: 10.1097/SLA.0b013e318277b625. [DOI] [PubMed] [Google Scholar]
- 12.Lambregts D.M., Vandecaveye V., Barbaro B. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol. 2011;18(August (8)):2224–2231. doi: 10.1245/s10434-011-1607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Paardt M.P., Zagers M.B., Beets-Tan R.G., Stoker J., Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology. 2013;269(October (1)):101–112. doi: 10.1148/radiol.13122833. [DOI] [PubMed] [Google Scholar]
- 14.Song I., Kim S.H., Lee S.J., Choi J.Y., Kim M.J., Rhim H. Value of diffusion-weighted imaging in the detection of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer: comparison with T2 weighted and PET/CT imaging. Br J Radiol. 2012;85(May (1013)):577–586. doi: 10.1259/bjr/68424021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ICRU Report 50 . International Commission for Radiation Units and Measurements; Bethesda: 1993. Prescribing, recording and reporting photon beam therapy; p. 71. [Google Scholar]
- 16.ICRU Report 62 . International Commission for Radiation Units and Measurements; Bethesda: 1999. Prescribing, recording and reporting photon beam therapy (Suppl. to ICRU Report 50) p. 52. [Google Scholar]
- 17.Glimelius B., Tiret E., Cervantes A., Arnold D., ESMO Guidelines Working Group Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(October (Suppl. 6)) doi: 10.1093/annonc/mdt240. vi81–8. [DOI] [PubMed] [Google Scholar]
- 18.Benson A.B., 3rd, Venook A.P., Bekaii-Saab T. Rectal Cancer, Version 2.2015. J Natl Compr Canc Netw. 2015;13(June (6)):719–728. doi: 10.6004/jnccn.2015.0087. [quiz 728] [DOI] [PubMed] [Google Scholar]
- 19.Lutz M.P., Zalcberg J.R., Glynne-Jones R. Second St. Gallen European Organisation for Research and Treatment of Cancer Gastrointestinal Cancer Conference: consensus recommendations on controversial issues in the primary treatment of rectal cancer. Eur J Cancer. 2016;August (63):11–24. doi: 10.1016/j.ejca.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Francois Y., Nemoz C.J., Baulieux J. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17(August (8)):2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 21.Bujko K., Nowacki M.P., Nasierowska-Guttmejer A., Michalski W., Bebenek M., Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93(October (10)):1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 22.Allegra C.J., Yothers G., O’Connell M.J. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst. 2015;107(September (11)) doi: 10.1093/jnci/djv248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park I.J., You Y.N., Agarwal A. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30(May (15)):1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tulchinsky H., Shmueli E., Figer A., Klausner J.M., Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15(October (10)):2661–2667. doi: 10.1245/s10434-008-9892-3. [DOI] [PubMed] [Google Scholar]
- 25.Kalady M.F., de Campos-Lobato L.F., Stocchi L. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250(October (4)):582–589. doi: 10.1097/SLA.0b013e3181b91e63. [DOI] [PubMed] [Google Scholar]
- 26.Moore H.G., Gittleman A.E., Minsky B.D. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47(March (3)):279–286. doi: 10.1007/s10350-003-0062-1. [DOI] [PubMed] [Google Scholar]
- 27.Zeng W.G., Zhou Z.X., Liang J.W. Impact of interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer on surgical and oncologic outcome. J Surg Oncol. 2014;110(September (4)):463–467. doi: 10.1002/jso.23665. [DOI] [PubMed] [Google Scholar]
- 28.Kwak Y.K., Kim K., Lee J.H. Timely tumor response analysis after preoperative chemoradiotherapy and curative surgery in locally advanced rectal cancer: a multi-institutional study for optimal surgical timing in rectal cancer. Radiother Oncol. 2016;119(June (3)):512–518. doi: 10.1016/j.radonc.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Habr-Gama A., Perez R.O., Proscurshim I. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2006;10(December (10)):1319–1328. doi: 10.1016/j.gassur.2006.09.005. [discussion 1328–9] [DOI] [PubMed] [Google Scholar]
- 31.Maas M., Beets-Tan R.G., Lambregts D.M. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29(December (35)):4633–4640. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 32.Al-Sukhni E., Milot L., Fruitman M. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19(July (7)):2212–2223. doi: 10.1245/s10434-011-2210-5. [DOI] [PubMed] [Google Scholar]
- 33.MERCURY Study Group Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(October (7572)):779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown G., Richards C.J., Bourne M.W. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227(May (2)):371–377. doi: 10.1148/radiol.2272011747. [DOI] [PubMed] [Google Scholar]
- 35.MERCURY Study Group, Shihab O.C., Taylor F. Relevance of magnetic resonance imaging-detected pelvic sidewall lymph node involvement in rectal cancer. Br J Surg. 2011;98(December (12)):1798–1804. doi: 10.1002/bjs.7662. [DOI] [PubMed] [Google Scholar]
- 36.Gollub M.J., Gultekin D.H., Akin O. Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol. 2012;22(April (4)):821–831. doi: 10.1007/s00330-011-2321-1. [DOI] [PubMed] [Google Scholar]
- 37.Lambregts D.M., Maas M., Riedl R.G. Value of ADC measurements for nodal staging after chemoradiation in locally advanced rectal cancer-a per lesion validation study. Eur Radiol. 2011;21(February (2)):265–273. doi: 10.1007/s00330-010-1937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulkarni T., Gollins S., Maw A., Hobson P., Byrne R., Widdowson D. Magnetic resonance imaging in rectal cancer downstaged using neoadjuvant chemoradiation: accuracy of prediction of tumour stage and circumferential resection margin status. Colorectal Dis. 2008;10(June (5)):479–489. doi: 10.1111/j.1463-1318.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 39.Suppiah A., Hunter I.A., Cowley J. Magnetic resonance imaging accuracy in assessing tumour down-staging following chemoradiation in rectal cancer. Colorectal Dis. 2009;11(March (3)):249–253. doi: 10.1111/j.1463-1318.2008.01593.x. [DOI] [PubMed] [Google Scholar]
- 40.Figueiras R.G., Goh V., Padhani A.R. The role of functional imaging in colorectal cancer. AJR. 2010;195:54–66. doi: 10.2214/AJR.10.4422. [DOI] [PubMed] [Google Scholar]
- 41.Tong T., Sun Y., Gollub M.J. Dynamic contrast-enhanced MRI: use in predicting pathological complete response to neoadjuvant chemoradiation in locally advanced rectal cancer. J Magn Reson Imaging. 2015;42(September (3)):673–680. doi: 10.1002/jmri.24835. [DOI] [PubMed] [Google Scholar]
- 42.Lim J.S., Kim D., Baek S.E. Perfusion MRI for the prediction of treatment response after preoperative chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2012;22(August (8)):1693–1700. doi: 10.1007/s00330-012-2416-3. [DOI] [PubMed] [Google Scholar]
- 43.Kim S.H., Lee J.M., Gupta S.N., Han J.K., Choi B.I. Dynamic contrast-enhanced MRI to evaluate the therapeutic response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer. J Magn Reson Imaging. 2014;40(September (3)):730–737. doi: 10.1002/jmri.24387. [DOI] [PubMed] [Google Scholar]
- 44.Korcakova E., Mirka H., Kastner J., Novak P., Svoboda T., Daum O. Evaluation of rectal carcinoma response to neoadjuvant treatment using multiparametric imaging on 3T MRI scanner. Cesk Radiol. 2015;69(3):165–173. [Google Scholar]