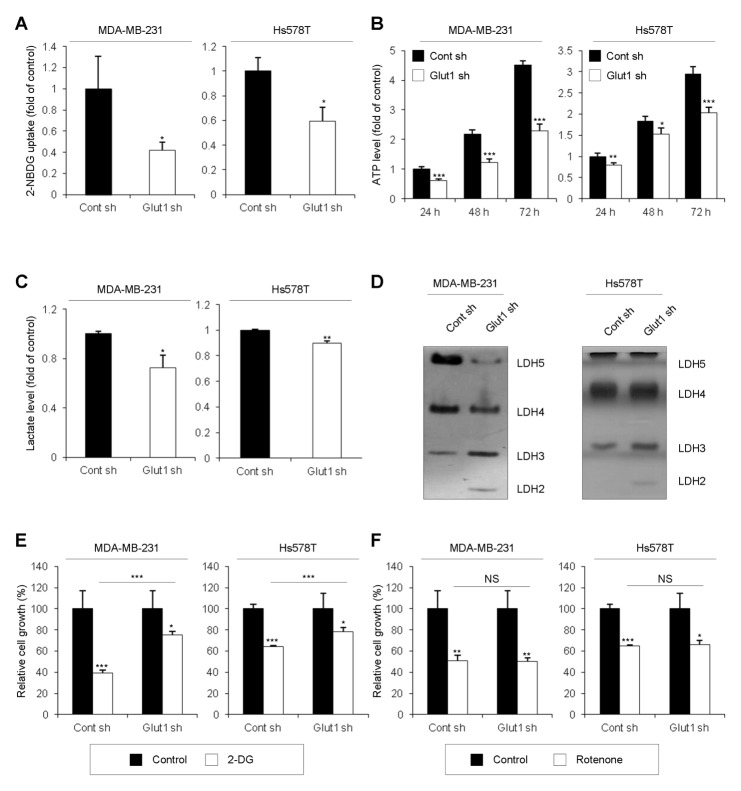
Fig. 2.
Reduction of glycolytic phenotypes by Glut1 knockdown. (A) For glucose uptake assays, Cont sh and Glut1 sh cells were seeded at 5 × 103 cells/well in 96-well plates, and glucose uptake was measured by 2-NBDG fluorescence using a plate reader (*P < 0.05). (B) For ATP assays, Cont sh and Glut1 sh cells were seeded at 5 × 103 cells/well in 96-well plates. After 24, 48, or 72 h, 100 μl of CellTiter-Glo® Reagent was added to each well. Cellular ATP levels were measured by luminescence (*P < 0.05, **P < 0.005, ***P < 0.0005). (C) For lactate production assays, Cont sh and Glut1 sh cells were seeded at 1 × 106 cells/100-mm dish. After 24 h, the culture medium was replaced with FBS-free DMEM. After a further 8 h, culture medium was harvested and lactate levels were analyzed by colorimetric assay (*P < 0.05, **P < 0.005). (D) For LDH zymography assays, 30-μg protein samples from each cell line were subjected to native gel electrophoresis. (E, F) Cont sh and Glut1 sh cells were seeded at 2 × 103 cells/well in 96-well plates and treated with 20 mM 2-deoxyglucose (2-DG) (E) or 20 μM rotenone (F) for 24 h before analysis by MTT assay (*P < 0.05, **P < 0.005, ***P < 0.0005).