Abstract
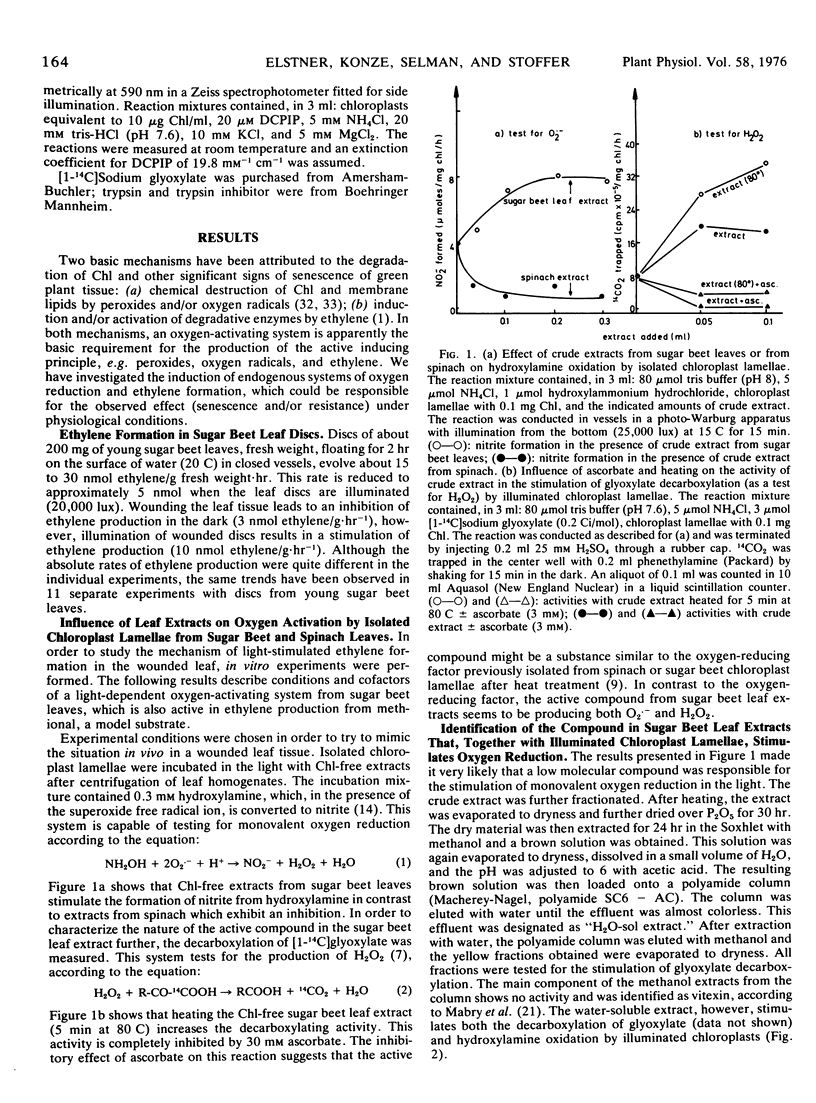
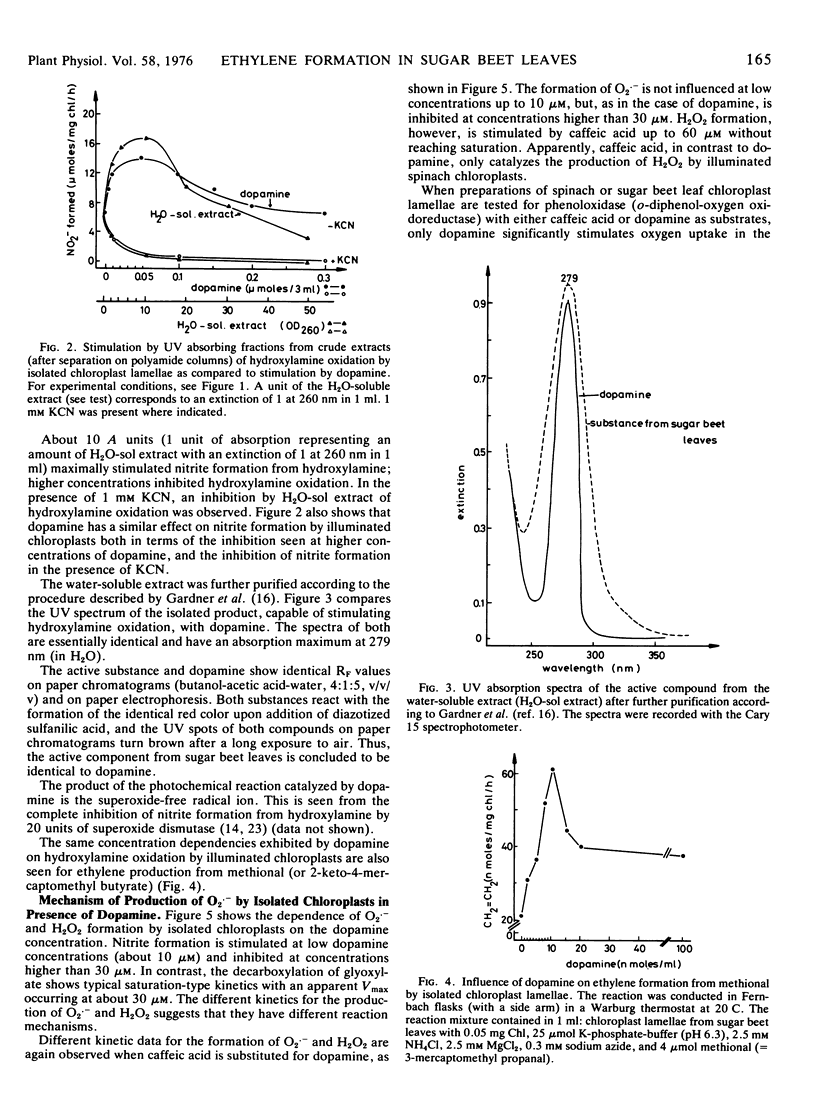
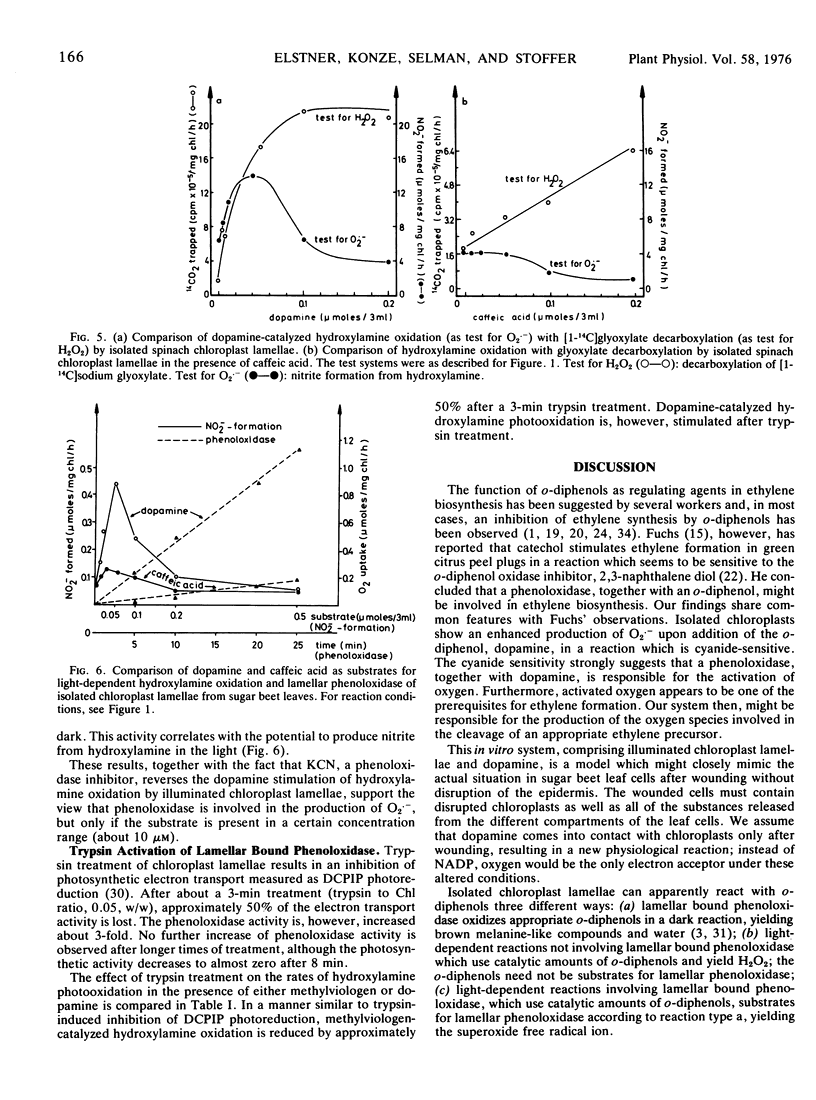
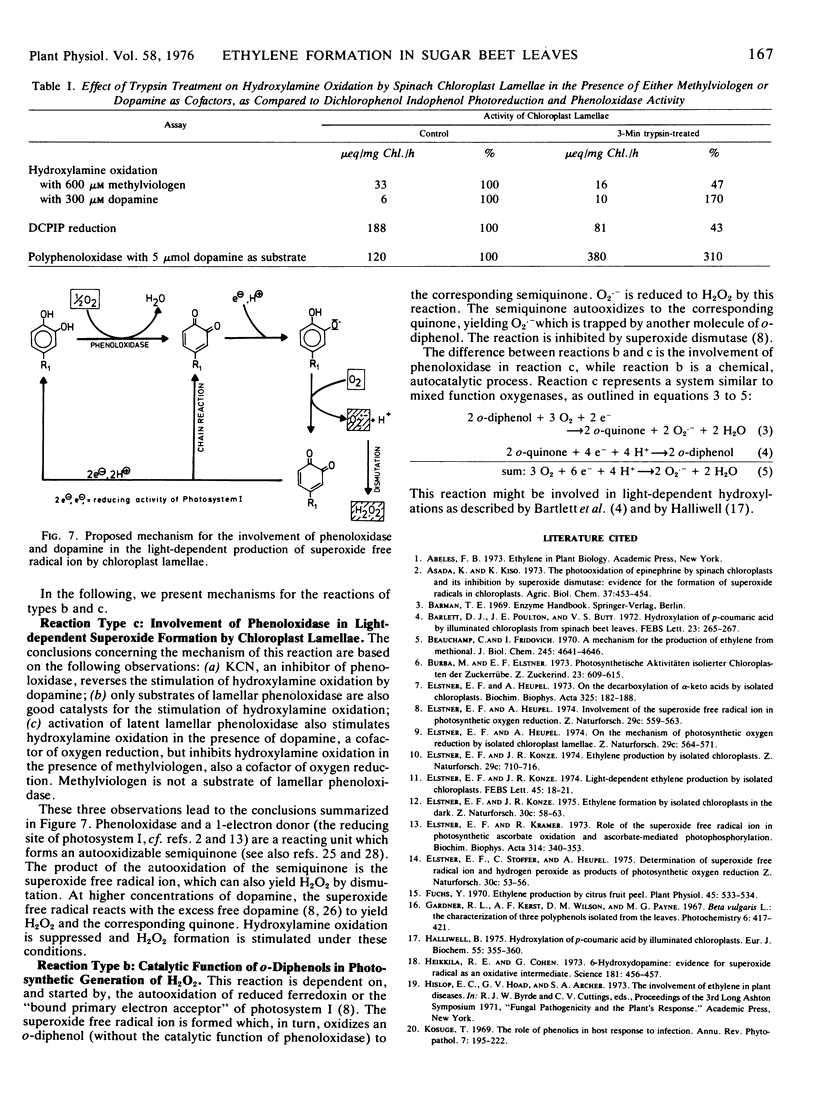
Ethylene production by sugar beet (Beta vulgaris L.) leaf discs is inhibited by white (or red, >610 nm) light or by wounding. In contrast, in wounded leaf discs, ethylene production is stimulated by light. The effect of light on wounded leaf discs has been studied by using an in vitro system which mimics the loss of compartmentation in the wounded leaf. Chlorophyll-free extracts from sugar beet leaves stimulate the production of the superoxide free radical ion (as a prerequisite for ethylene formation) by illuminated chloroplast lamellae. The substance from the crude leaf extracts which is active in stimulating the production of the superoxide free radical ion has been identified as 3-hydroxytyramine (dopamine). Exogenous dopamine between 5 μm and 100 μm stimulates ethylene formation by illuminated chloroplast lamellae from methional. It also stimulates the production of the superoxide free radical ion, the formation of which apparently involves both a lamellar phenoloxidase and photosynthetic electron transport as a 1-electron donor, and is cyanide-sensitive.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Elstner E. F., Heupel A. Involvement of the superoxide free radical ion in photosynthetic oxygen reduction. Z Naturforsch C. 1974 Sep-Oct;29C(9-10):559–563. doi: 10.1515/znc-1974-9-1019. [DOI] [PubMed] [Google Scholar]
- Elstner E. F., Heupel A. On the decarboxylation of alpha-keto acids by isolated chloroplasts. Biochim Biophys Acta. 1973 Oct 19;325(1):182–188. doi: 10.1016/0005-2728(73)90164-3. [DOI] [PubMed] [Google Scholar]
- Elstner E. F., Heupel A. On the mechanism of photosynthetic oxygen reduction by isolated chloroplast lamellae. Z Naturforsch C. 1974 Sep-Oct;29C(9-10):564–571. doi: 10.1515/znc-1974-9-1020. [DOI] [PubMed] [Google Scholar]
- Elstner E. F., Kramer R. Role of the superoxide free radical ion in photosynthetic ascorbate oxidation and ascorbate-mediated photophosphorylation. Biochim Biophys Acta. 1973 Sep 26;314(3):340–353. doi: 10.1016/0005-2728(73)90118-7. [DOI] [PubMed] [Google Scholar]
- Elstner E., Konze J. R. Light-dependent ethylene production by isolated chloroplasts. FEBS Lett. 1974 Sep 1;45(1):18–21. doi: 10.1016/0014-5793(74)80800-8. [DOI] [PubMed] [Google Scholar]
- Fuchs Y. Ethylene production by citrus fruit peel: stimulation by phenol derivatives. Plant Physiol. 1970 Apr;45(4):533–534. doi: 10.1104/pp.45.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Hydroxylation of p-Coumaric acid by illuminated chloroplasts. The role of superoxide. Eur J Biochem. 1975 Jul 1;55(2):355–360. doi: 10.1111/j.1432-1033.1975.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Cohen G. 6-Hydroxydopamine: evidence for superoxide radical as an oxidative intermediate. Science. 1973 Aug 3;181(4098):456–457. doi: 10.1126/science.181.4098.456. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Miller R. W., Macdowall F. D. The tiron free radical as a sensitive indicator of chloroplastic photoautoxidation. Biochim Biophys Acta. 1975 Apr 14;387(1):176–187. doi: 10.1016/0005-2728(75)90062-6. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May 25;247(10):3170–3175. [PubMed] [Google Scholar]
- Nelson N., Drechsler Z., Neumann J. Photophosphorylation in digitonin subchloroplast particles. Absence of a light-induced pH shift. J Biol Chem. 1970 Jan 10;245(1):143–151. [PubMed] [Google Scholar]
- Sawada Y., Oyama T., Yamazaki I. Preparation and physicochemical properties of green pea superoxide dismutase. Biochim Biophys Acta. 1972 May 12;268(2):305–312. doi: 10.1016/0005-2744(72)90325-7. [DOI] [PubMed] [Google Scholar]
- Selman B. R., Bannister T. T. Trypsin inhibition of photosystem II. Biochim Biophys Acta. 1971 Dec 7;253(2):428–436. doi: 10.1016/0005-2728(71)90046-6. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E. Activation of polyphenol oxidase of chloroplasts. Plant Physiol. 1973 Feb;51(2):234–244. doi: 10.1104/pp.51.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. D. Role of superoxide radicals in the lipid peroxidation of intracellular membranes. FEBS Lett. 1975 Mar 1;51(1):180–183. doi: 10.1016/0014-5793(75)80882-9. [DOI] [PubMed] [Google Scholar]
- Yang S. F. Biosynthesis of ethylene. Ethylene formation from methional by horseradish peroxidase. Arch Biochem Biophys. 1967 Nov;122(2):481–487. doi: 10.1016/0003-9861(67)90222-6. [DOI] [PubMed] [Google Scholar]
- Yang S. F. Further studies on ethylene formation from alpha-keto-gamma-methylthiobutyric acid or beta-methylthiopropionaldehyde by peroxidase in the presence of sulfite and oxygen. J Biol Chem. 1969 Aug 25;244(16):4360–4365. [PubMed] [Google Scholar]


