Abstract
Citrullination, the post-translational conversion of arginines to citrullines, may contribute to rheumatoid arthritis development given the generation of anti-citrullinated protein antibodies (ACPAs). However, it is not known which peptidylarginine deiminase (PAD) catalyzes the citrullination seen in inflammation. PAD4 exacerbates inflammatory arthritis and is critical for neutrophil extracellular traps (NETs). NETs display citrullinated antigens targeted by ACPAs and thus may be a source of citrullinated protein. However, PAD4 is not required for citrullination in inflamed lungs. PAD2 is important for citrullination in healthy tissues and is present in NETs, but its role in citrullination in the inflamed joint, NETosis and inflammatory arthritis is unknown. Here we use mice with TNFα-induced inflammatory arthritis, a model of rheumatoid arthritis, to identify the roles of PAD2 and PAD4 in citrullination, NETosis, and arthritis. In mice with TNFα-induced arthritis, citrullination in the inflamed ankle was increased as determined by western blot. This increase was unchanged in the ankles of mice that lack PAD4. In contrast, citrullination was nearly absent in the ankles of PAD2-deficient mice. Interestingly, PAD2 was not required for NET formation as assessed by immunofluorescence or for killing of Candida albicans as determined by viability assay. Finally, plasma cell numbers as assessed by flow cytometry, IgG levels quantified by ELISA, and inflammatory arthritis as determined by clinical and pathological scoring were all reduced in the absence of PAD2. Thus, PAD2 contributes to TNFα-induced citrullination and arthritis, but is not required for NETosis. In contrast, PAD4, which is critical for NETosis, is dispensable for generalized citrullination supporting the possibility that NETs may not be a major source of citrullinated protein in arthritis.
Keywords: Citrullination, Peptidylarginine deiminase, TNFα, Neutrophil extracellular trap, Rheumatoid arthritis, Murine model
1. Introduction
Citrullination, the post-translational conversion of arginines in a protein to citrullines, has gained attention related to the observation that anti-citrullinated protein antibodies (ACPAs) can be detected in about 75% of people with rheumatoid arthritis [1]. Thus, protein citrullination (and similar post-translational modifications) may be a trigger in genetically susceptible individuals for the initial loss of tolerance seen in rheumatoid arthritis. Once tolerance is lost, inflammation increases, inflammatory cytokines like TNFα rise, and clinical rheumatoid arthritis develops.
Although ACPAs are a hallmark of rheumatoid arthritis, citrullination increases in a variety of inflamed tissues [2] including the rheumatoid joint [3]. Peptidylarginine deiminases (PADs) catalyze citrullination, but which PAD catalyzes inflammation-induced citrullination in rheumatoid arthritis and other diseases is unclear. Single nucleotide polymorphisms (SNPs) in PADI2 and PADI4, the genes that encode PAD2 and PAD4, have been associated with rheumatoid arthritis [4–12], suggesting that they may both be involved in disease pathogenesis. Further, both PAD2 and PAD4 are found in immune cells [13–15] as well as in the rheumatoid joint [16, 17] and a pan-PAD inhibitor reduces collagen induced arthritis [18]. Also, both PAD2 and PAD4 can hypercitrullinate proteins during immune-mediated membranolysis, which has been implicated in ACPA formation [19]. Adding complexity, both PAD4 and PAD2 can autocitrullinate [20–22], which may regulate their activity in disease. Thus, PAD2, PAD4, or both could citrullinate proteins in inflamed areas. Understanding the individual contributions of the PADs is important since inhibiting specific PAD enzymes might prove useful in treating rheumatoid arthritis. Indeed, this is an active area of investigation by both academic and commercial research labs with several isoform-specific inhibitors generated to date [23, 24].
There is additional evidence that PAD4 may be important for citrullination in rheumatoid arthritis. PAD4 contributes to inflammation and arthritis in three different murine models of rheumatoid arthritis [25–27]. Also, PAD4 is required for LPS-induced histone citrullination and neutrophil extracellular trap (NET) formation [28, 29] and NETs display some of the same citrullinated antigens that are targeted by ACPAs [30]. Together, these findings suggest that PAD4 may contribute to the increased citrullination seen in arthritic joints. However, no reduction in serum [25] or lung [31] protein citrullination is seen in TNFα-induced arthritis when PAD4 is absent.
Thus, PAD2 may be the major contributor to joint citrullination in inflammatory arthritis. In support of this idea, synovial fluid PAD2 levels correlate with overall PAD activity as well as disease activity in rheumatoid arthritis [32]. Moreover, PAD2-deficient mice have a reduction in PAD activity in healthy tissues as well as reduced citrullination in the central nervous system in experimental autoimmune encephalomyelitis [33, 34]. Also, PAD2 displays less restrictive substrate specificity than PAD4 [35], which could lead to citrullination of more arginines. Finally, PAD2 is present on NETs [36] and is required to citrullinate histones in breast cancer [37], but it is not known if PAD2 is required for citrullination in inflamed joints, NETosis or arthritis.
Here we use mice with TNFα-induced arthritis to determine if PAD2 or PAD4 is required for citrullination in inflamed joints and if PAD2 is required for NETosis and inflammatory arthritis.
2. Material and Methods
2.1. Animals
Tg3647 mice, which overexpress systemically one copy of the TNFα transgene (TNF+ mice) [38], were crossed with PAD4-deficient mice [28] to generate TNF+PAD4+/+ and TNF+PAD4−/− mice. TNF+ mice were also crossed with PAD2-deficient mice [34] to generate TNF+PAD2+/+ and TNF+PAD2−/− mice. Littermates were used for all experiments and sex matched whenever possible. Approximately equal numbers of male and female mice were used for experiments. PAD2 and PAD4 were confirmed to be deleted. Mice were maintained in pathogen-free conditions and all murine experiments were approved by the University of Wisconsin Animal Care and Use Committee.
2.2. Protein lysates
One ankle joint per mouse was harvested, flash frozen and homogenized using a Bullet Blender (Next Advance, Averill Park, USA) in RIPA buffer (Sigma Aldrich, St. Louis, USA) supplemented with protease inhibitor cocktail (Sigma Aldrich). Lysates were sonicated briefly and total protein in the supernatants was quantified.
2.3. Western blots to detect citrullination
Equal amounts of protein were subjected to gel electrophoresis using two polyacrylamide gels. One gel was stained with brilliant blue G colloidal solution (Sigma Aldrich) and the other was transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, USA) using a semi-dry blotting system (Bio-Rad). The membrane was then subjected to the Anti-Citrulline (Modified) Detection Kit (AMC kit) (Millipore Sigma) to visualize citrullinated proteins. Imaging was performed using ImageQuant LAS 4000 (GE Healthcare, Madison, USA), followed by densitometry using Quantity One 1–D Analysis Software (Bio-Rad). Brilliant blue G stained gels were imaged using an Odyssey infrared scanner (LI-COR Biosciences, Lincoln, USA), followed by densitometry. AMC signal was normalized to total protein as detected by brilliant blue and blots were normalized to each other for overall signal intensity. Blots treated with the AMC kit without primary antibody showed no signal (data not shown).
2.4. Western blots to detect PAD2
Equal amounts of protein were subjected to polyacrylamide gel electrophoresis followed by transfer to PVDF membrane as above. The membrane was blocked with 5% milk, incubated at 4°C overnight with rabbit anti-actin a ntibody (Sigma Aldrich) and anti-PAD2 mouse monoclonal IgG (clone DN2) [39] diluted 1:100 and 1:200 respectively in 2.5% milk, washed, incubated with anti-mouse IgG conjugated to IRDye 680LT and anti-rabbit IgG conjugated to IRDye 800LT (LI-COR Biosciences, Lincoln, NE, USA), and washed. Blots were imaged using the Odyssey infrared scanner followed by densitometry.
2.5. Neutrophil purification
Femurs and tibias were flushed using RPMI1640 (Thermo Fisher Scientific, Waltham, USA) with 2% fetal bovine serum (FBS) (Thermo Fisher Scientific) and 2 mM EDTA (Sigma Aldrich). Neutrophils were purified using EasySep Mouse Neutrophil Enrichment Kit (Stemcell Technologies, Vancouver, Canada) according to the manufacturer’s instructions. Purity of neutrophils was confirmed by flow cytometry to be 92%.
2.6. Quantitative polymerase chain reaction (qPCR)
RNA was isolated using Trizol reagent according to the manufacturer’s instructions (Thermo Fisher Scientific) followed by treatment with DNase I (Sigma Aldrich). cDNA was synthesized using iScript cDNA synthesis kit according to the manufacturer’s instructions (Bio-Rad). qPCR was performed on equal amounts of cDNA using iTaq Universal SYBR Green Supermix (Bio-Rad) on a StepOnePlus Real-Time PCR system (Thermo Fisher Scientific). Primers for PAD2 (forward: 5′-AGCAGCGGAGGGCTTAC-3′, reverse: 5′-CACGCGGCTCCCATACT-3′) and 18s (forward: 5′-GAATAATGGAATAGGACCGCGG-3′, reverse: 5′-GGAACTACGACGGTATCTGATC-3′) were designed using IDT PrimerQuest Tool. All genes were normalized to 18S rRNA and relative gene expression (fold change) was calculated by 2−ΔΔCt method.
2.7. Immunofluorescence staining and NET quantification
All chemicals, unless otherwise indicated, are from Sigma Aldrich. Purified neutrophils were treated with 100 ng/ml TNFα in RPMI for 30 minutes on poly-L-lysine coated glass coverslips, followed by the addition of 10 μg/ml lipopolysaccharide (LPS) and then incubation for 4 hours at 37°C with 5% CO2. Coverslips were fixed and permeabilized with a solution of 4% paraformaldehyde, 1% NP-40, and 0.5% Triton X-100 in phosphate buffered saline (PBS) for 30 minutes, washed, and blocked for 1.5 hours at room temperature with 2.5% bovine serum albumin (BSA), 5% goat serum, and 0.5% Tween-20 in PBS. The coverslips were then incubated for 1 hour with rabbit anti-histone H4 (citrulline 3) antibody (MilliporeSigma) diluted 1:200 in block solution, washed, incubated with a 1:200 dilution of donkey anti-rabbit TRITC and a 1:1000 dilution of 4′,6-diamidino-2-phenylindole (DAPI) in blocking solution for 30 minutes at room temperature, washed, and mounted using Aquamount (Thermo Fisher Scientific). Five predetermined locations on the coverslip were imaged using a Leica Fluorescence Microscope at 400x. The total number of neutrophils and NETs (defined as large, cloud-like masses with protrusions staining positive for DAPI and citrullinated histone H4) were counted manually in a blinded manner for each of the 5 images.
2.8. Candida killing assay
All chemicals, unless otherwise indicated, are from Sigma Aldrich. A modified XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) assay was performed [40, 41]. Candida albicans strain SC5314 [42] was stored in 15% glycerol stock at −80°C and maintained on yeast extract-peptone-dextrose (YPD) medium supplemented with uridine (1% yeast extract, 2% peptone, 2% dextrose medium, 0.08% uridine) prior to experiments. A single colony was used to propagate a culture in YPD with uridine at 30°C with orbital shaking at 220 RP M overnight. To obtain exponentially growing cells, cultures were diluted 20-fold and incubated for an additional 2 hours. Candida were collected by centrifugation, washed twice with PBS and adjusted to a concentration of 4×107 cells/ml in RPMI 1640 (without phenol red) supplemented with 2% FBS. Neutrophils were suspended at 4×106 cells/mL in identical culture media and pre-incubated with TNFα (100 ng/ml) for 30 minutes on ice. Next, 40μL of neutrophils or culture media and 4μl of Candida were added to each well of a 384 well clear plate (Corning, USA) with 100nM phorbol myristate acetate (PMA). To some wells, 10 μg/ml of cytochalasin D was added [43]. Plates were incubated at 37°C with 5% CO 2 for 4 hours. Neutrophils were then lysed for 15 minutes at 37°C in a final concentration of 0.03% TritonX-100. Background absorption at 492 nm was pre-read in a Synergy H1 microplate reader (BioTek, Winooski, USA) followed by the addition of 30 μL of 9:1 XTT working solution (0.75 mg/ml XTT in PBS with 2% glucose: phenazine methosulfate 0.32 mg/ml in ddH2O). Following a 25 minute incubation at 37°C in 5% CO2, absorption was read at 492 nm and the baseline absorbance was subtracted. Experiments were performed in triplicate. To determine percent killing values, neutrophils without Candida were subtracted from neutrophils with Candida and all were normalized to Candida alone.
2.9. Enzyme-linked immunosorbent assay (ELISA)
Sera were collected by cardiac puncture and subjected to a mouse IgG ELISA quantification kit according to manufacturer’s protocol (Bethyl Laboratories Inc., Montgomery, USA).
2.10. Flow cytometry
Red blood cells were lysed using a hypotonic NaCl solution. Remaining cells were suspended in MACS buffer (1% BSA, 2% FBS, 0.03% sodium azide and 2 mM EDTA) and stained using anti-mouse B220 conjugated to allophycocyanin (clone RA3-6B2, eBioscience, San Diego, USA) and anti-mouse CD138 conjugated to phycoerythrin (clone 281-2, BD Biosciences, San Jose, CA, USA). Samples were acquired using a BD FACSCalibur machine and data was analyzed using FlowJo software.
2.11. Clinical arthritis scoring
Scoring was performed in a blinded manner on a scale of 0–3 as previously described [25]. Scale: 0, no arthritis; 0.5, mild joint deformity and swelling; 1.0, moderate joint deformity and swelling; 1.5, moderate/severe joint deformity, moderate swelling, and reduced grip strength; 2.0, severe joint deformity, moderate swelling, and no grip strength; 2.5, severe joint deformity, moderate/severe swelling, and no grip strength; 3.0, severe joint deformity and swelling and no grip strength.
2.12. Pathology
Rear hind legs were fixed in 10% neutral buffered formalin, decalcified for 30 hours with Surgipath Decalcifier 1 (Leica Biosystems, Lincolnshire, USA), processed with a VIP processor, embedded in paraffin (Sakura Tissue Tek, Torrance, CA, USA), sectioned at 5 microns on a Leica microtome, and stained with hematoxylin and eosin (H&E). A board certified pathologist scored the severity of synovitis at the tibio-talar joint on a scale of 0–4 (0, none; 1, mild; 2, mild to moderate; 3, moderate; 4, severe) and determined the percentage of the surface of the tibio-talar joint that was eroded in a blinded manner.
2.12. Statistics
All data was analyzed using a paired t-test with the p value reported as significant if less than or equal to 0.05.
3. Results
3.1. Joint citrullination is increased in TNFα-induced arthritis without a significant requirement for PAD4
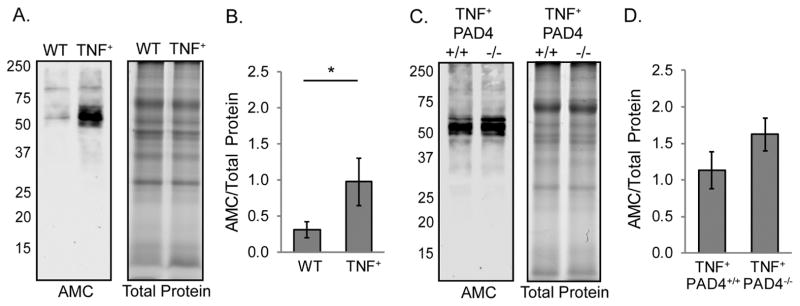
TNF+ mice develop an inflammatory, destructive arthritis very similar to rheumatoid arthritis [38]. Protein lysates from the ankles of wild type and TNF+ mice were assessed for total protein citrullination by western blot using an anti-modified citrulline detection kit. As shown in Figure 1A and 1B, generalized protein citrullination was increased in TNF+ compared to wild type joints. To determine if PAD4 is required for joint protein citrullination in TNFα-induced arthritis, we prepared ankle lysates from TNF+PAD4+/+ and TNF+PAD4−/− mice for western blot as above. There was no loss of citrullination in TNF+PAD4−/− compared to TNF+PAD4+/+ ankles (Figure 1C and 1D). Therefore, PAD4 is not required for gross protein citrullination in the arthritic joints of TNFα-overexpressing mice as detected by the anti-modified citrulline detection kit.
Figure 1. Joint citrullination is increased in TNFα overexpressing mice without a requirement for PAD4.
Ankle protein lysates from 4–5 month old TNF+ and littermate control (WT) mice were subjected to western blot to detect citrullinated proteins and gel electrophoresis to detect total protein. A. Representative blot (left) and gel (right). B. The density of the entire lanes was determined and citrullinated protein signal was normalized to total protein signal. Average and SEM are graphed (n=9, *p<0.05). Ankle lysates from 4–5 month old TNF+PAD4+/+ and TNF+PAD4−/− littermates were similarly assessed. C. Representative blot (left) and gel (right). D. Graph with average and SEM (n=8).
3.2. PAD2 is required for joint citrullination in TNFα-induced arthritis
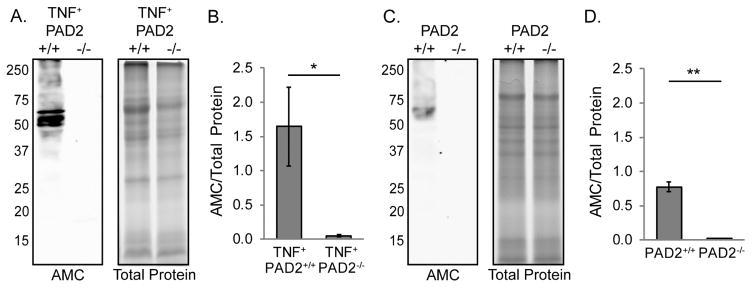
To determine the requirement for PAD2 in joint citrullination, we assessed total protein citrullination by western blot as above in arthritic ankles from TNF+PAD2+/+ and TNF+PAD2−/− mice. As shown in Figure 2A and 2B, there was a dramatic loss of protein citrullination in TNF+PAD2−/− compared to TNF+PAD2+/+ arthritic ankles. Since PAD activity in multiple tissues is lost in PAD2-deficient mice [33], we hypothesized that PAD2 also would be responsible for citrullination in the non-inflamed joint. Thus, we assessed protein citrullination by western blot as above in the ankles of PAD2+/+ and PAD2−/− mice that do not overexpress TNFα. As shown in Figure 2C and 2D, citrullination was significantly reduced in non-inflamed PAD2−/− ankles. Taken together, these data suggest that PAD2 is required for baseline and TNFα-induced citrullination in the joint.
Figure 2. PAD2 is required for joint citrullination.
Protein lysates from the ankles of 4–5 month old TNF+PAD2+/+ and TNF+PAD2−/− mice were subjected to western blot to detect citrullinated proteins and gel electrophoresis to detect total protein. A. Representative blot (left) and gel (right). B. The density of the entire lanes was determined and citrullinated protein signal was normalized to total protein signal. Average and SEM are graphed for the entire lane (TNF+PAD2+/+ n=9, TNF+PAD2−/− n=8, *p<0.05). Ankle lysates from PAD2+/+ and PAD2−/− littermates were similarly assessed. C. Representative blot (left) and gel (right). D. Graph with average and SEM (PAD2+/+ n=4, PAD2−/− n=5, **p<0.01).
3.3 PAD2 is not increased in inflamed joints in the absence of PAD4
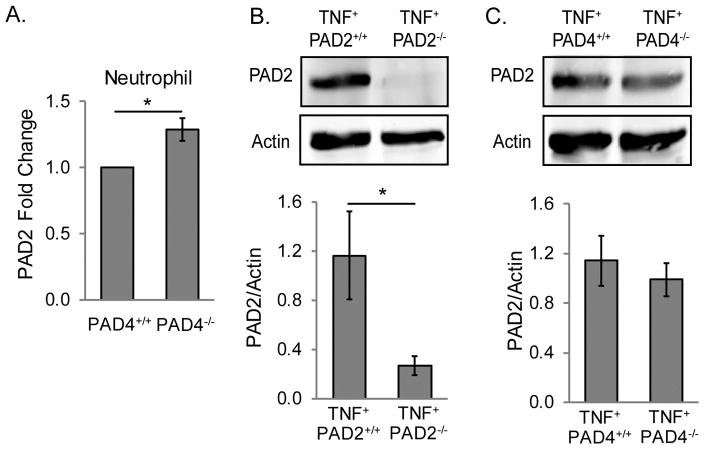
PAD2 expression has been shown to be increased in the absence of PAD4 in neutrophils [26]. Therefore, we hypothesized that the unaltered citrullination in PAD4-deficient arthritic joints might be due to upregulation of PAD2. To test this hypothesis, first we purified neutrophils from PAD4+/+ and PAD4−/− mice and assessed PAD2 expression by qPCR. As shown in Figure 3A, PAD2 was only mildly upregulated in PAD4-deficient neutrophils. Although modest, these levels are significantly different. We then used a monoclonal antibody against PAD2 [39] to determine if PAD2 is increased in arthritic joints in the absence of PAD4. As a control, we subjected ankle lysates from TNF+PAD2+/+ and TNF+PAD2−/− mice to western blot to detect PAD2 (Figure 3B). We then assessed PAD2 protein levels in TNF+PAD4+/+ and TNF+PAD4−/− arthritic ankles and found no significant increase in PAD2 in TNF+PAD4−/− compared to TNF+PAD4+/+ ankles (Figure 3C). Thus, upregulation of PAD2 does not appear to explain the unaltered levels of citrullination in TNF+PAD4−/− ankles.
Figure 3. PAD2 is not increased in inflamed joints in the absence of PAD4, but is upregulated in PAD4-deficient neutrophils.
A. RNA from PAD4+/+ and PAD4−/− neutrophils was subjected to qPCR to assess PAD2 gene expression levels with average and SEM graphed for the fold change (n=6). B. Protein lysates from the ankles of TNF+PAD2+/+ and TNF+PAD2−/− mice were assessed for PAD2 protein by western blot. Representative blots (upper panel) and average with SEM for quantification of PAD2 normalized to actin in the lower panel (TNF+PAD2+/+ n=9, TNF+PAD2−/− n=8). C. Protein lysates from the ankles of TNF+PAD4+/+ and TNF+PAD4−/− mice were assessed for PAD2 protein by western blot. Representative blots (upper panel) and average with SEM for quantification of PAD2 normalized to actin in the lower panel (n=7). For all panels, *p<0.05.
3.4. PAD2 is not required for NETosis
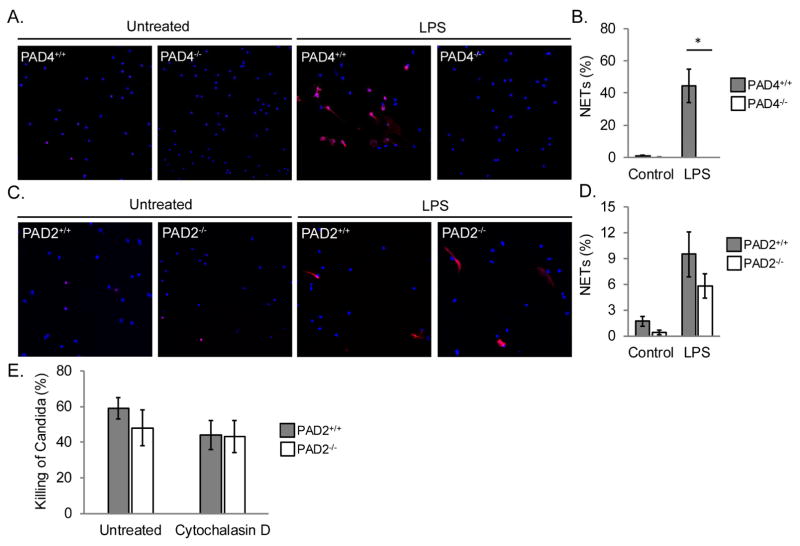
Given the loss of citrullination in the inflamed joints of PAD2-deficient mice, we hypothesized that PAD2 might be required for NETosis. To test this hypothesis, we purified neutrophils from PAD2+/+ and PAD2−/− mice, stimulated with TNFα and LPS, performed immunofluorescent staining, and quantified NETosis. As a control, PAD4+/+ and PAD4−/− neutrophils were treated identically. As shown in Figure 4A and 4B, PAD4−/− neutrophils were unable to make NETs as expected [28]. In contrast, PAD2−/− neutrophils were able to make NETs (Figure 4C and 4D). NETs can kill a variety of pathogens including Candida albicans [41, 44]. To test if PAD2−/− neutrophils could kill Candida, we subjected PAD2+/+ and PAD2−/− neutrophils to a modified XTT assay. As shown in Figure 4E, PAD2−/− neutrophils could effectively kill Candida regardless of whether phagocytosis was blocked with cytochalasin D or not. Taken together, these data suggest that PAD2 is not required for neutrophils to form NETs or kill Candida.
Figure 4. PAD2 is not required for NETosis.
Neutrophils from PAD4+/+ and PAD4−/− mice were untreated or treated with TNFα and LPS followed by staining for citrullinated histone H4 (red) and DNA (blue). A. Representative images at 400x. B. Graph with average and SEM for the percent of cells that formed NETs (n=3, *p=0.05). C. Neutrophils from PAD2+/+ and PAD2−/− mice were treated identically to the PAD4+/+ and PAD4−/− neutrophils. C. Representative images at 400x. D. Graph with average and SEM for the percent of cells that formed NETs (n=5). E. PAD2+/+ and PAD2−/− neutrophils were incubated with Candida in the presence or absence of Cytochalasin D. The percent of Candida killed was detected by XTT. Average and SEM are graphed (n = 3).
3.5. PAD2 is required for plasma cells and IgG in TNFα-induced arthritis
NETosis occurs in the absence of PAD2, but other immune functions could be impaired in PAD2-deficient mice. TNF+PAD4−/− mice have reduced IgG [25]. Therefore, we tested if PAD2 was also required for IgG levels in TNFα-induced arthritis. IgG was quantified for TNF+PAD2+/+ and TNF+PAD2−/− sera by ELISA. As shown in Figure 5A, TNF+PAD2−/− mice had reduced serum IgG compared to TNF+PAD2+/+ mice. Of note, female TNFα-overexpressing mice tend to have higher levels of IgG, but the reduction in IgG in the absence of PAD2 was independent of the sex of the mice.
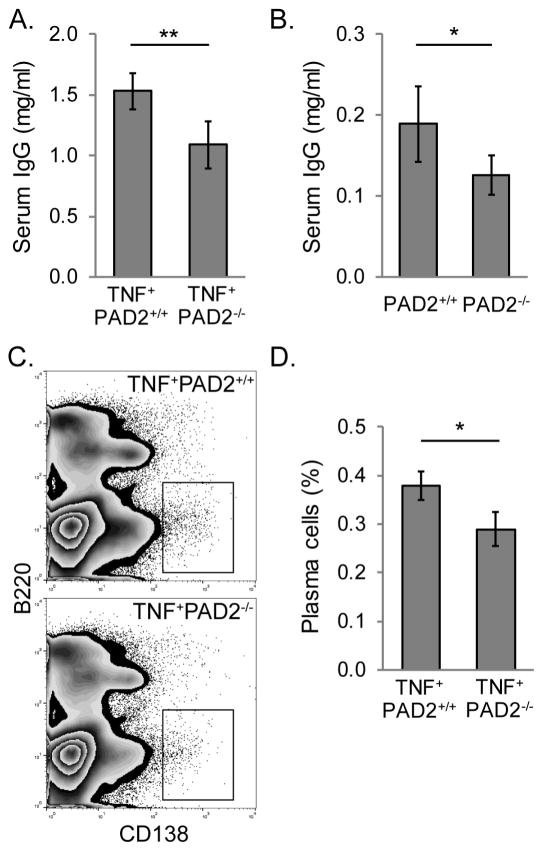
Figure 5. PAD2 contributes to plasma cells and IgG levels in TNFα-induced arthritis.
A. Total serum IgG was quantified for 4–5 month old TNF+PAD2+/+ and TNF+PAD2−/− mice by ELISA with average and SEM graphed (n=8, **p<0.01). B. Total serum IgG was quantified for 2 month old PAD2+/+ and PAD2−/− mice by ELISA with average and SEM graphed (n=7, *p<0.05). Bone marrow from TNF+PAD2+/+ and TNF+PAD2−/− mice was stained for B220 and CD138 and subjected to flow cytometry. C. Representative zebra plots of live cells. D. Average and SEM for the percentage of B220LOCD138HI plasma cells is graphed (n=9, *p<0.05).
We have previously shown that PAD4-deficient mice that do not overexpress TNFα have normal levels of IgG [25]. Thus, we determined if serum IgG levels in PAD2-deficient mice in the absence of TNFα overexpression were normal. As shown in Figure 5B, despite an overall lower level of IgG in mice that do not overexpress TNFα, PAD2−/− mice have reduced total IgG compared to PAD2+/+ mice. Therefore, PAD2 appears to be required to achieve the IgG levels seen in arthritic and non-arthritic mice.
Since IgG is primarily made by B220LOCD138HI plasma cells in the bone marrow [45], we determined if bone marrow plasma cells were reduced in TNF+PAD2−/− mice by flow cytometry. As shown in Figure 5C and 5D, bone marrow plasma cells were, indeed, reduced in TNF+PAD2−/− compared to TNF+PAD2+/+ mice. These data suggest that PAD2 is required for normal plasma cell numbers and IgG levels in TNFα-induced arthritis.
3.6. PAD2 is required for maximal severity of TNFα-induced arthritis
We have previously shown that PAD4 exacerbates TNFα-induced arthritis [25]. To determine if PAD2 contributes to TNFα-induced arthritis, we clinically scored arthritis severity in TNF+PAD2+/+ and TNF+PAD2−/− mice based on joint swelling, joint deformity and grip strength monthly [25]. We saw no difference in arthritis severity at 2 and 3 months of age (Figure 6A). However, at 4 months of age, there was a significant, although modest, reduction in arthritis in TNF+PAD2−/− compared to TNF+PAD2+/+ mice (Figure 6A). Since PAD2 is expressed in muscle [46], we were concerned that TNF+PAD2−/− mice might have reduced grip strength due to a role for PAD2 in muscle making arthritis scores higher for PAD2-deficient mice unrelated to inflammatory arthritis severity. Therefore, we also calculated clinical arthritis scores using only joint swelling and deformity. As shown in Figure 6A, arthritis scores were reduced at both 3 and 4 months of age in TNF+PAD2−/− compared to TNF+PAD2+/+ mice when grip strength was not included in the score. Finally, we scored the joints histologically for the percent erosion of the tibio-talar joint surface as well as the extent of synovitis. As shown in Figure 6B–D, there was reduced erosion and synovitis of the tibio-talar joint in TNF+PAD2−/− compared to TNF+PAD2+/+ mice. Together, our data suggest that PAD2 is required for maximal severity of TNFα-induced arthritis.
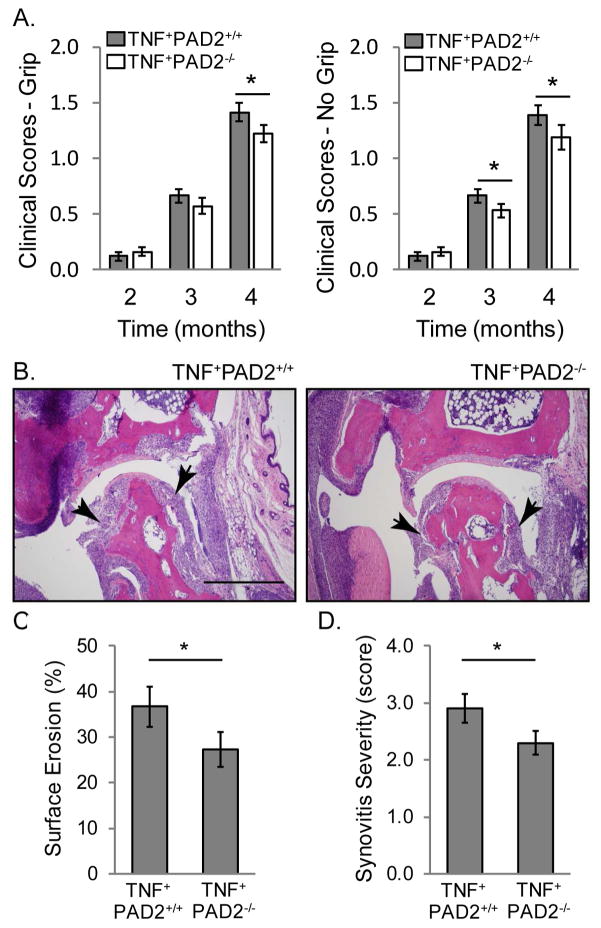
Figure 6. PAD2 is required for TNFα-induced inflammatory arthritis.
A. Severity of clinical arthritis was scored on a scale of 0–3 in 2, 3, and 4 month old TNF+PAD2+/+ and TNF+PAD2−/− mice including (left) or not including (right) grip strength with average and SEM graphed (2 and 3 months: n=25 TNF+PAD2+/+ and n=24 TNF+PAD2−/− mice; 4 months: n=19 pairs; *p<0.05). The ankles of 4–5 month old TNF+PAD2+/+ and TNF+PAD2−/− mice were fixed, sectioned, and stained with H&E. B. Representative images with arrows indicating inflamed synovium/pannus invading the tibio-talar joint (Magnification 100x, bar 500 μm). The percentage of surface erosion (C) and extent of synovitis on a scale of 0–4 (D) at the tibio-talar joint was determined in a blinded manner with average and SEM graphed (TNF+PAD2+/+ n=11, TNF+PAD2−/− n=10, *p<0.05).
4. Discussion
As expected, we found that TNFα overexpression leads to increased citrullination in inflamed joints consistent with the finding that TNFα drives citrullination in the serum [25] and lung [31]. TNFα may also drive citrullination in the human rheumatoid joint providing fodder for continued inflammation particularly in ACPA+ individuals. If true, this would provide further support for a circular model of rheumatoid arthritis pathogenesis in which citrullination contributes to the formation of ACPAs, which deposit in the joint and activate immune cells to produce TNFα and other cytokines, which then induce citrullination to fuel the fire.
However, despite the known requirement for PAD4 in histone citrullination [28, 29], PAD4 does not appear to be required for generalized TNFα-induced joint citrullination as detected by the anti-modified citrulline antibody. This method likely does not detect every citrullinated protein, but use of the citrulline probe rhodamine-phenylglyoxal (Rh-PG) [47] is limited by extensive background and use of the mouse monoclonal anti-peptidylcitrulline antibody F95 [48] is limited by binding of the anti-mouse IgM secondary antibody in murine joint tissue (data not shown). Nonetheless, our finding is consistent with the observation that PAD4 is not required for TNFα-induced lung [31] or serum [25] protein citrullination, as determined by other methods. Thus, we conclude that PAD4 is not required for at least a significant portion of the increased citrullination seen in TNFα-induced arthritis.
A role for PAD4 in TNFα-induced arthritis [25] without a clear requirement for generalized protein citrullination suggests that PAD4 may contribute to rheumatoid arthritis apart from antigen citrullination. This idea is consistent with the finding that genetic variants in PADI4 associate with both ACPA+ and ACPA− rheumatoid arthritis [49] as well as ulcerative colitis [50], an inflammatory disorder that does not involve ACPAs. Further, PAD4 contributes to IgG levels and T cell activation [25, 27]. Thus, even though PAD4 is a citrullinating enzyme and protein citrullination is increased in inflammation, PAD4 may contribute to rheumatoid arthritis apart from the generation of citrullinated antigens.
In this manuscript, we have shown a role for PAD2 in TNFα-induced arthritis, providing the first evidence that PAD2 contributes to inflammatory arthritis. The reduction in arthritis that we detect in the absence of PAD2 is modest. Previously, we demonstrated that PAD4 contributes to TNFα-induced arthritis at 5 months of age, with little to no effect at younger ages [25]. Unfortunately, TNF+ mice, either with or without PAD2, die earlier for unclear reasons in our current animal facility than in our previous facility. Thus, we were unable to score arthritis consistently at 5 months of age. It is possible that if we had been able to score arthritis regularly at 5 months, we would have detected an even larger reduction in arthritis in PAD2-deficient mice. Also, mice with TNFα-induced arthritis do not produce classic ACPAs [25, 51]. Given the requirement for PAD2 in generating citrullinated protein, one might expect an even larger role for PAD2 in ACPA-dependent arthritis, which could be the case in human rheumatoid arthritis.
The reduced arthritis in PAD2-deficient mice may be related to a reduction in plasma cells and IgG levels, especially since PAD2 is not required for NETosis. Interestingly, TNF+PAD4−/− mice have a reduction in serum IgG levels, but not plasma cells [25]. It is possible that plasma cells or serum IgG are reduced at least in part secondary to overall reduced inflammation in the PAD-deficient mice. Alternatively, the PADs may regulate plasma cells and antibodies. PAD2 and PAD4 may be important within B lineage cells for regulating IgG production or other pathways, although expression data for the PADs in B cells is conflicted [9, 14, 15, 52], or important in cells that regulate B cells. For example, PAD2 is upregulated in the bone marrow mesenchymal stem cells of patients with multiple myeloma, a plasma cell malignancy, driving production of IL-6, a molecule that supports plasma cells [53][54]. Perhaps in the absence of PAD2, IL-6 is reduced in the bone marrow microenvironment leading to reduced plasma cells and IgG levels. Further work is needed to clarify the role of PAD2 and PAD4 in the regulation of plasma cells and antibodies.
Finally, we show that PAD2 is required for joint citrullination in TNFα–induced arthritis. If the relative contributions of PAD2 and PAD4 to citrullination that we observed in mice apply to humans, then PAD2 may be responsible for the bulk of citrullinated proteins targeted by ACPAs in rheumatoid arthritis. Interestingly, despite the requirement for PAD2 in TNFα-induced joint citrullination and the observation that NETs are source of citrullinated antigens [30], PAD2 is not required for NETosis in our experiments. In contrast, PAD4, which is critical for NETosis [28, 29], does not play a major role in gross citrullination in TNFα-induced arthritis. Together, these findings suggest that NETs may not be the main source of citrullinated protein in arthritic mice. Perhaps the main source of citrullinated proteins in TNFα-driven arthritis, and potentially rheumatoid arthritis given the high levels of TNFα, is immune-mediated membranolysis-induced hypercitrullination, which can be catalyzed by PAD2 [19]. It is impossible to directly extrapolate our findings to human disease. Nonetheless, even if NETs do not provide the majority of citrullinated proteins in rheumatoid arthritis, they may still be important for generating and/or displaying key citrullinated antigens targeted by ACPAs.
5.0 Conclusion
Our data support an important role for PAD2 in citrullination and arthritis severity in TNFα-induced inflammatory arthritis without a major role in NETosis suggesting that NETs may not be the main source of citrullinated protein in rheumatoid arthritis.
Highlights.
PAD4 is dispensable for citrullination in TNFα-induced arthritis.
PAD2 is required for citrullination in TNFα-induced arthritis.
PAD2 is dispensable for NET formation.
PAD2 is required for maximal TNFα-induced IgG and inflammatory arthritis.
Acknowledgments
This work was supported by the Rheumatology Research Foundation via a Scientist Development Award and a National Institute of Health (NIH) K08 AR065500 to MAS as well as a NIH K08 AI108727 and Burroughs Wellcome Fund 1012299 to JEN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Venrooij WJ, van Beers JJ, Pruijn GJ. Anti-CCP antibodies: the past, the present and the future. Nat Rev Rheumatol. 2011;7:391–8. doi: 10.1038/nrrheum.2011.76. [DOI] [PubMed] [Google Scholar]
- 2.Makrygiannakis D, af Klint E, Lundberg IE, Lofberg R, Ulfgren AK, Klareskog L, et al. Citrullination is an inflammation-dependent process. Ann Rheum Dis. 2006;65:1219–22. doi: 10.1136/ard.2005.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinloch A, Lundberg K, Wait R, Wegner N, Lim NH, Zendman AJ, et al. Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum. 2008;58:2287–95. doi: 10.1002/art.23618. [DOI] [PubMed] [Google Scholar]
- 4.Hou S, Gao GP, Zhang XJ, Sun L, Peng WJ, Wang HF, et al. PADI4 polymorphisms and susceptibility to rheumatoid arthritis: a meta-analysis. Mod Rheumatol. 2013;23:50–60. doi: 10.1007/s10165-012-0639-4. [DOI] [PubMed] [Google Scholar]
- 5.Kang CP, Lee HS, Ju H, Cho H, Kang C, Bae SC. A functional haplotype of the PADI4 gene associated with increased rheumatoid arthritis susceptibility in Koreans. Arthritis Rheum. 2006;54:90–6. doi: 10.1002/art.21536. [DOI] [PubMed] [Google Scholar]
- 6.Gandjbakhch F, Fajardy I, Ferre B, Dubucquoi S, Flipo RM, Roger N, et al. A functional haplotype of PADI4 gene in rheumatoid arthritis: positive correlation in a French population. J Rheumatol. 2009;36:881–6. doi: 10.3899/jrheum.080398. [DOI] [PubMed] [Google Scholar]
- 7.Panati K, Pal S, Rao KV, Reddy VD. Association of single nucleotide polymorphisms (SNPs) of PADI4 gene with rheumatoid arthritis (RA) in Indian population. Genes Genet Syst. 2012;87:191–6. doi: 10.1266/ggs.87.191. [DOI] [PubMed] [Google Scholar]
- 8.Plenge RM, Padyukov L, Remmers EF, Purcell S, Lee AT, Karlson EW, et al. Replication of putative candidate-gene associations with rheumatoid arthritis in >4,000 samples from North America and Sweden: association of susceptibility with PTPN22, CTLA4, and PADI4. Am J Hum Genet. 2005;77:1044–60. doi: 10.1086/498651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, Suzuki M, et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet. 2003;34:395–402. doi: 10.1038/ng1206. [DOI] [PubMed] [Google Scholar]
- 10.Takata Y, Inoue H, Sato A, Tsugawa K, Miyatake K, Hamada D, et al. Replication of reported genetic associations of PADI4, FCRL3, SLC22A4 and RUNX1 genes with rheumatoid arthritis: results of an independent Japanese population and evidence from meta-analysis of East Asian studies. J Hum Genet. 2008;53:163–73. doi: 10.1007/s10038-007-0232-4. [DOI] [PubMed] [Google Scholar]
- 11.Too CL, Murad S, Dhaliwal JS, Larsson P, Jiang X, Ding B, et al. Polymorphisms in peptidylarginine deiminase associate with rheumatoid arthritis in diverse Asian populations: evidence from MyEIRA study and meta-analysis. Arthritis Res Ther. 2012;14:R250. doi: 10.1186/ar4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang X, Xia Y, Pan J, Meng Q, Zhao Y, Yan X. PADI2 is significantly associated with rheumatoid arthritis. PLoS One. 2013;8:e81259. doi: 10.1371/journal.pone.0081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darrah E, Rosen A, Giles JT, Andrade F. Peptidylarginine deiminase 2, 3 and 4 have distinct specificities against cellular substrates: novel insights into autoantigen selection in rheumatoid arthritis. Ann Rheum Dis. 2012;71:92–8. doi: 10.1136/ard.2011.151712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang X, Yamada R, Suzuki A, Sawada T, Yoshino S, Tokuhiro S, et al. Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology (Oxford) 2005;44:40–50. doi: 10.1093/rheumatology/keh414. [DOI] [PubMed] [Google Scholar]
- 15.Vossenaar ER, Radstake TR, van der Heijden A, van Mansum MA, Dieteren C, de Rooij DJ, et al. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis. 2004;63:373–81. doi: 10.1136/ard.2003.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foulquier C, Sebbag M, Clavel C, Chapuy-Regaud S, Al Badine R, Mechin MC, et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007;56:3541–53. doi: 10.1002/art.22983. [DOI] [PubMed] [Google Scholar]
- 17.Makrygiannakis D, Revu S, Engstrom M, Af Klint E, Nicholas AP, Pruijn GJ, et al. Local administration of glucocorticoids decrease synovial citrullination in rheumatoid arthritis. Arthritis Res Ther. 2012;14:R20. doi: 10.1186/ar3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, et al. N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186:4396–404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero V, Fert-Bober J, Nigrovic PA, Darrah E, Haque UJ, Lee DM, et al. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci Transl Med. 2013;5:209ra150. doi: 10.1126/scitranslmed.3006869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrade F, Darrah E, Gucek M, Cole RN, Rosen A, Zhu X. Autocitrullination of human peptidyl arginine deiminase type 4 regulates protein citrullination during cell activation. Arthritis Rheum. 2010;62:1630–40. doi: 10.1002/art.27439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewallen DM, Bicker KL, Subramanian V, Clancy KW, Slade DJ, Martell J, et al. Chemical Proteomic Platform To Identify Citrullinated Proteins. ACS Chem Biol. 2015;10:2520–8. doi: 10.1021/acschembio.5b00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blachere NE, Parveen S, Frank MO, Dill BD, Molina H, Orange DE. High titer rheumatoid arthritis antibodies preferentially bind fibrinogen citrullinated by peptidyl arginine deiminase 4. Arthritis Rheumatol. 2016 doi: 10.1002/art.40035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian V, Knight JS, Parelkar S, Anguish L, Coonrod SA, Kaplan MJ, et al. Design, synthesis, and biological evaluation of tetrazole analogs of Cl-amidine as protein arginine deiminase inhibitors. J Med Chem. 2015;58:1337–44. doi: 10.1021/jm501636x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11:189–91. doi: 10.1038/nchembio.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shelef MA, Sokolove J, Lahey LJ, Wagner CA, Sackmann EK, Warner TF, et al. Peptidylarginine Deiminase 4 Contributes to Tumor Necrosis Factor alpha-Induced Inflammatory Arthritis. Arthritis Rheumatol. 2014;66:1482–91. doi: 10.1002/art.38393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki A, Kochi Y, Shoda H, Seri Y, Fujio K, Sawada T, et al. Decreased severity of experimental autoimmune arthritis in peptidylarginine deiminase type 4 knockout mice. BMC Musculoskelet Disord. 2016;17:205. doi: 10.1186/s12891-016-1055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seri Y, Shoda H, Suzuki A, Matsumoto I, Sumida T, Fujio K, et al. Peptidylarginine deiminase type 4 deficiency reduced arthritis severity in a glucose-6-phosphate isomerase-induced arthritis model. Sci Rep. 2015;5:13041. doi: 10.1038/srep13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–62. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One. 2011;6:e22043. doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bawadekar M, Gendron-Fitzpatrick A, Rebernick R, Shim D, Warner TF, Nicholas AP, et al. Tumor necrosis factor alpha, citrullination, and peptidylarginine deiminase 4 in lung and joint inflammation. Arthritis Res Ther. 2016;18:173. doi: 10.1186/s13075-016-1068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damgaard D, Senolt L, Nielsen CH. Increased levels of peptidylarginine deiminase 2 in synovial fluid from anti-CCP-positive rheumatoid arthritis patients: Association with disease activity and inflammatory markers. Rheumatology (Oxford) 2016;55:918–27. doi: 10.1093/rheumatology/kev440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Beers JJ, Zendman AJ, Raijmakers R, Stammen-Vogelzangs J, Pruijn GJ. Peptidylarginine deiminase expression and activity in PAD2 knock-out and PAD4-low mice. Biochimie. 2013;95:299–308. doi: 10.1016/j.biochi.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 34.Raijmakers R, Vogelzangs J, Raats J, Panzenbeck M, Corby M, Jiang H, et al. Experimental autoimmune encephalomyelitis induction in peptidylarginine deiminase 2 knockout mice. J Comp Neurol. 2006;498:217–26. doi: 10.1002/cne.21055. [DOI] [PubMed] [Google Scholar]
- 35.Assohou-Luty C, Raijmakers R, Benckhuijsen WE, Stammen-Vogelzangs J, de Ru A, van Veelen PA, et al. The human peptidylarginine deiminases type 2 and type 4 have distinct substrate specificities. Biochim Biophys Acta. 2014;1844:829–36. doi: 10.1016/j.bbapap.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 36.Spengler J, Lugonja B, Jimmy Ytterberg A, Zubarev RA, Creese AJ, Pearson MJ, et al. Release of Active Peptidyl Arginine Deiminases by Neutrophils Can Explain Production of Extracellular Citrullinated Autoantigens in Rheumatoid Arthritis Synovial Fluid. Arthritis Rheumatol. 2015;67:3135–45. doi: 10.1002/art.39313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Bolt M, Guertin MJ, Chen W, Zhang S, Cherrington BD, et al. Peptidylarginine deiminase 2-catalyzed histone H3 arginine 26 citrullination facilitates estrogen receptor alpha target gene activation. Proc Natl Acad Sci U S A. 2012;109:13331–6. doi: 10.1073/pnas.1203280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douni E, Akassoglou K, Alexopoulou L, Georgopoulos S, Haralambous S, Hill S, et al. Transgenic and knockout analyses of the role of TNF in immune regulation and disease pathogenesis. Journal of inflammation. 1995;47:27–38. [PubMed] [Google Scholar]
- 39.Damgaard D, Palarasah Y, Skjodt K, Catrina AI, Hensen SM, Pruijn GJ, et al. Generation of monoclonal antibodies against peptidylarginine deiminase 2 (PAD2) and development of a PAD2-specific enzyme-linked immunosorbent assay. J Immunol Methods. 2014;405:15–22. doi: 10.1016/j.jim.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Nett JE, Cain MT, Crawford K, Andes DR. Optimizing a Candida biofilm microtiter plate model for measurement of antifungal susceptibility by tetrazolium salt assay. Journal of clinical microbiology. 2011;49:1426–33. doi: 10.1128/JCM.02273-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson CJ, Cabezas-Olcoz J, Kernien JF, Wang SX, Beebe DJ, Huttenlocher A, et al. The Extracellular Matrix of Candida albicans Biofilms Impairs Formation of Neutrophil Extracellular Traps. PLoS Pathog. 2016;12:e1005884. doi: 10.1371/journal.ppat.1005884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E.coli pyrF mutations. Mol Gen Genet. 1984;198:179–82. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 43.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cellular microbiology. 2006;8:668–76. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 44.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–76. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–42. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 46.Takahara H, Oikawa Y, Sugawara K. Purification and characterization of peptidylarginine deiminase from rabbit skeletal muscle. J Biochem. 1983;94:1945–53. doi: 10.1093/oxfordjournals.jbchem.a134548. [DOI] [PubMed] [Google Scholar]
- 47.Bicker KL, Subramanian V, Chumanevich AA, Hofseth LJ, Thompson PR. Seeing citrulline: development of a phenylglyoxal-based probe to visualize protein citrullination. J Am Chem Soc. 2012;134:17015–8. doi: 10.1021/ja308871v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicholas AP, Whitaker JN. Preparation of a monoclonal antibody to citrullinated epitopes: its characterization and some applications to immunohistochemistry in human brain. Glia. 2002;37:328–36. [PubMed] [Google Scholar]
- 49.Bang SY, Han TU, Choi CB, Sung YK, Bae SC, Kang C. Peptidyl arginine deiminase type IV (PADI4) haplotypes interact with shared epitope regardless of anti-cyclic citrullinated peptide antibody or erosive joint status in rheumatoid arthritis: a case control study. Arthritis Res Ther. 2010;12:R115. doi: 10.1186/ar3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen CC, Isomoto H, Narumi Y, Sato K, Oishi Y, Kobayashi T, et al. Haplotypes of PADI4 susceptible to rheumatoid arthritis are also associated with ulcerative colitis in the Japanese population. Clin Immunol. 2008;126:165–71. doi: 10.1016/j.clim.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–81. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakashima K, Arai S, Suzuki A, Nariai Y, Urano T, Nakayama M, et al. PAD4 regulates proliferation of multipotent haematopoietic cells by controlling c-myc expression. Nat Commun. 2013;4:1836. doi: 10.1038/ncomms2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jourdan M, Cren M, Robert N, Bollore K, Fest T, Duperray C, et al. IL-6 supports the generation of human long-lived plasma cells in combination with either APRIL or stromal cell-soluble factors. Leukemia. 2014;28:1647–56. doi: 10.1038/leu.2014.61. [DOI] [PubMed] [Google Scholar]
- 54.McNee G, Eales KL, Wei W, Williams DS, Barkhuizen A, Bartlett DB, et al. Citrullination of histone H3 drives IL-6 production by bone marrow mesenchymal stem cells in MGUS and multiple myeloma. Leukemia. 2016 doi: 10.1038/leu.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]