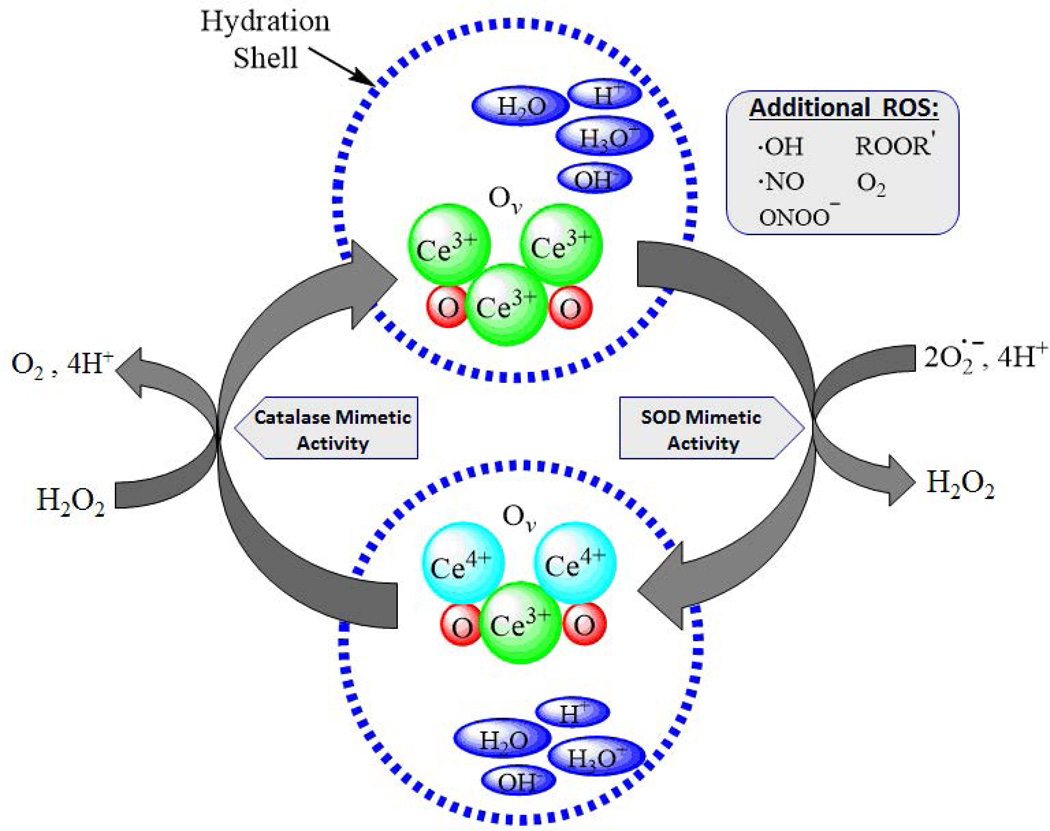
Figure 1. Hypothesized Mechanism of Action of CeONPs.
In a given CeONP, the cerium atom exists in the 3+ and 4+ valence states, bound to oxygen and containing oxygen vacancies (Ov). When exposed to a superoxide radical, it exhibits SOD-mimetic activity, and Ce3+ is converted to Ce4+, with a corresponding change in oxygen vacancies. There is also likely a contribution to this reaction from the hydration shell around the CeONP. Superoxide is converted to H2O2. Via a catalase-mimetic activity involving Ce4+, H2O2 is converted to O2 + 4H+, and cerium valence to +3 (with corresponding changes in oxygen vacancies), regenerating the origin CeONP state. Again, there is a likely contribution from ions present in the water hydration shell. In the biological milieu, this action exists in a continuous cycle, depending on the ionic species exposed to the CeONPs, the hydration shell, oxygen partial pressure, and any surrounding ionic species. Although we utilized superoxide and H2O2 as examples, radicals scavenged could be any number of biologically relevant free radicals.