Abstract
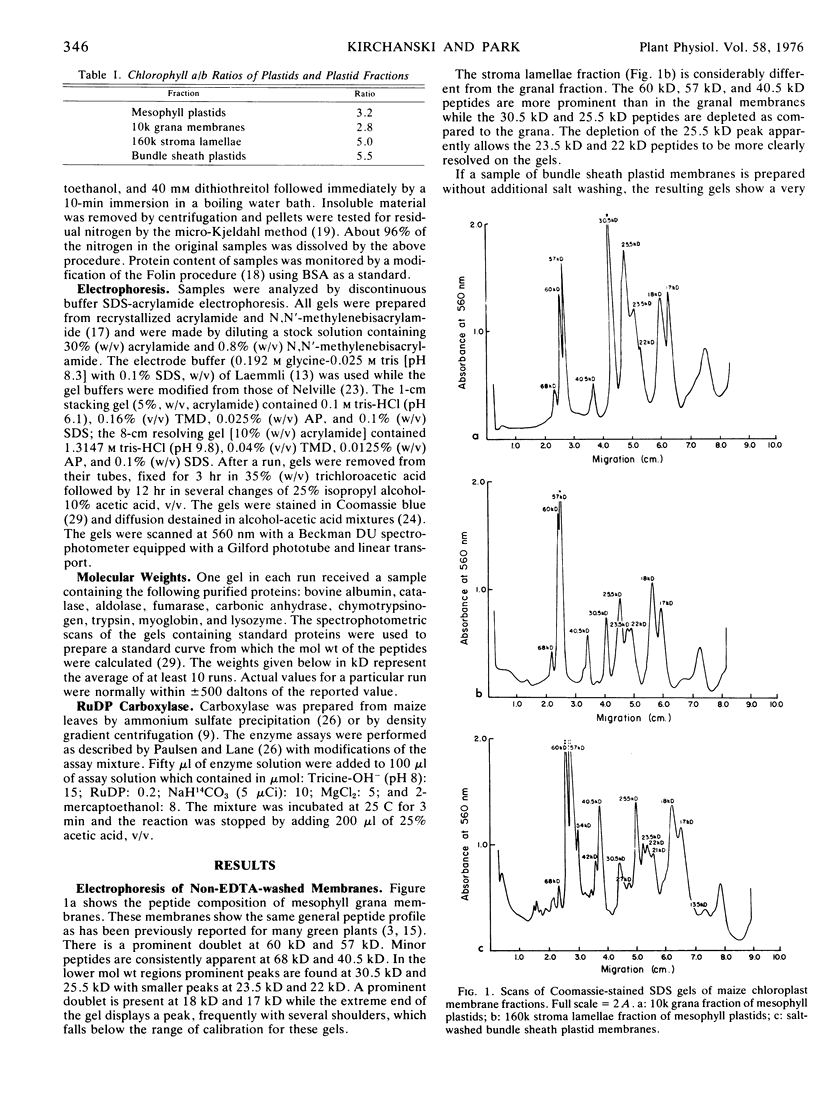
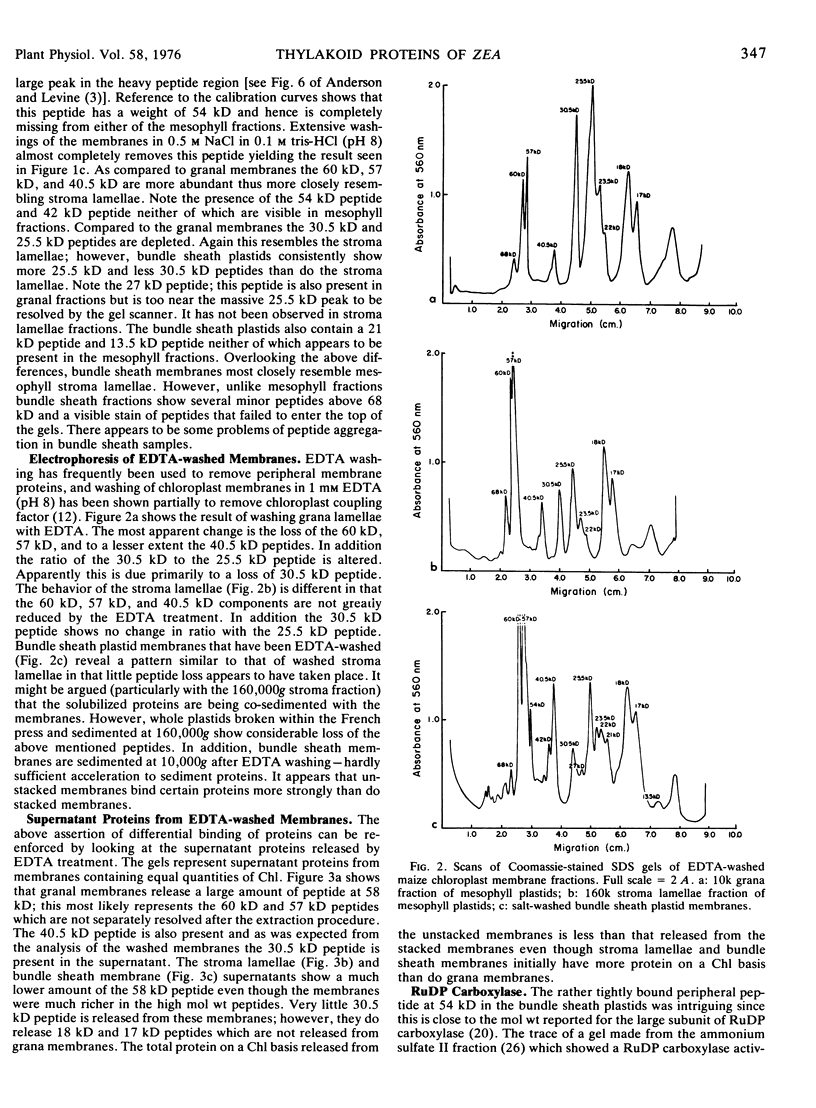
The proteins from both grana and stroma lamellae of maize (Zea mays) mesophyll plastids and from maize bundle sheath plastid membranes have been compared by electrophoresis in sodium dodecyl sulfate-polyacrylamide gels using a discontinuous buffer system. Peptide differences between grana and stroma lamellae were essentially quantitative and not qualitative. Bundle sheath plastid membrane peptides more closely resembled those of the ultrastructurally similar stroma lamellae. However, bundle sheath membranes contained several peptides not apparent in the stroma lamellae.
The unappressed membranes (stroma lamellae and bundle sheath plastid membranes) were enriched in heavy (60-40 kilodaltons) peptides and depleted in light (31-20 kilodaltons) peptides as compared to stacked grana membranes. The heavier peptides were tentatively identified as subunits of chloroplast coupling factor. These peptides in unappressed membranes were much more resistant to removal by washing with ethylenediaminetetraacetate (under conditions of low ionic strength) than they were in granal membranes.
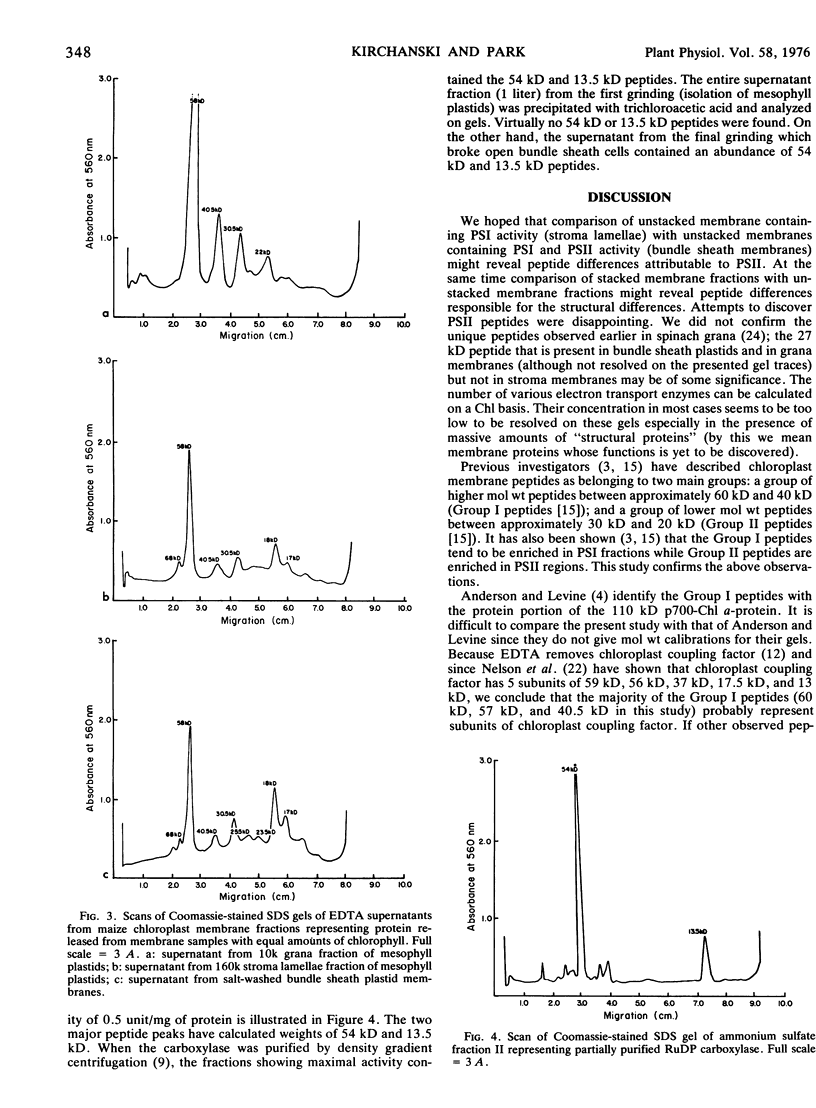
Ribulose-1,5-diphosphate carboxylase was identified on the gels and was localized exclusively in the bundle sheath cells. It is suggested that sodium dodecyl sulfate electrophoresis is a simple method to test for the localization of carboxylase in various C4 plastid fractions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. S., Bain J. M., Bishop D. G., Smillie R. M. Photosystem II Activity in Agranal Bundle Sheath Chloroplasts from Zea mays. Plant Physiol. 1972 Apr;49(4):461–466. doi: 10.1104/pp.49.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. M., Levine R. P. The relationship between chlorophyll-protein complexes and chloroplast membrane polypeptides. Biochim Biophys Acta. 1974 Jul 25;357(1):118–126. doi: 10.1016/0005-2728(74)90117-0. [DOI] [PubMed] [Google Scholar]
- Anderson J. M. The molecular organization of chloroplast thylakoids. Biochim Biophys Acta. 1975 Aug 15;416(2):191–235. doi: 10.1016/0304-4173(75)90007-5. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. J., Gross E. L. Protein-protein interactions of light-harvesting pigment protein from spinach chloroplasts. I.Ca-2+ binding and its relation to protein association. Biochim Biophys Acta. 1975 Jun 17;387(3):557–567. doi: 10.1016/0005-2728(75)90093-6. [DOI] [PubMed] [Google Scholar]
- Genge S., Pilger D., Hiller R. G. The relationship between chlorophyll b and pigment-protein complex II. Biochim Biophys Acta. 1974 Apr 23;347(1):22–30. doi: 10.1016/0005-2728(74)90196-0. [DOI] [PubMed] [Google Scholar]
- Goldthwaite J. J., Bogorad L. A one-step method for the isolation and determination of leaf ribulose-1,5-diphosphate carboxylase. Anal Biochem. 1971 May;41(1):57–66. doi: 10.1016/0003-2697(71)90191-6. [DOI] [PubMed] [Google Scholar]
- Goodenough U. W., Armstrong J. J., Levine R. P. Photosynthetic Properties of ac-31, a Mutant Strain of Chlamydomonas reinhardi Devoid of Chloroplast Membrane Stacking. Plant Physiol. 1969 Jul;44(7):1001–1012. doi: 10.1104/pp.44.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques F., Park R. B. Further Chemical and Morphological Characterization of Chloroplast Membranes from a Chlorophyll b-less Mutant of Hordeum vulgare. Plant Physiol. 1975 Apr;55(4):763–767. doi: 10.1104/pp.55.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. H., Moudrianakis E. N. Hill reaction site in chloroplast membranes: non-participation of the quantasome particle in photoreduction. J Mol Biol. 1967 Jul 28;27(2):323–333. doi: 10.1016/0022-2836(67)90023-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine R. P., Burton W. G., Duram H. A. Membrane polypeptides associated with photochemical systems. Nat New Biol. 1972 Jun 7;237(75):176–177. doi: 10.1038/newbio237176a0. [DOI] [PubMed] [Google Scholar]
- Lien S., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. 8. Properties of silicotungstate-treated subchloroplast particles. J Biol Chem. 1971 Jul 10;246(13):4298–4307. [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Packer L. The role of cations in the organization of chloroplast membranes. Arch Biochem Biophys. 1971 Sep;146(1):337–347. doi: 10.1016/s0003-9861(71)80072-3. [DOI] [PubMed] [Google Scholar]
- Nelson N., Deters D. W., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. 8. Properties of isolated subunits of coupling factor 1 from spinach chloroplasts. J Biol Chem. 1973 Mar 25;248(6):2049–2055. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Nolan W. G., Park R. B. Comparative studies on the polypeptide composition of chloroplast lamellae and lamellar fractions. Biochim Biophys Acta. 1975 Feb 14;375(3):406–421. doi: 10.1016/0005-2736(75)90356-9. [DOI] [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Sane P. V., Goodchild D. J., Park R. B. Characterization of chloroplast photosystems 1 and 2 separated by a non-detergent method. Biochim Biophys Acta. 1970 Aug 4;216(1):162–178. doi: 10.1016/0005-2728(70)90168-4. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woo K. C., Anderson J. M., Boardman N. K., Downton W. J., Osmond C. B., Thorne S. W. Deficient Photosystem II in Agranal Bundle Sheath Chloroplasts of C(4) Plants. Proc Natl Acad Sci U S A. 1970 Sep;67(1):18–25. doi: 10.1073/pnas.67.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]


