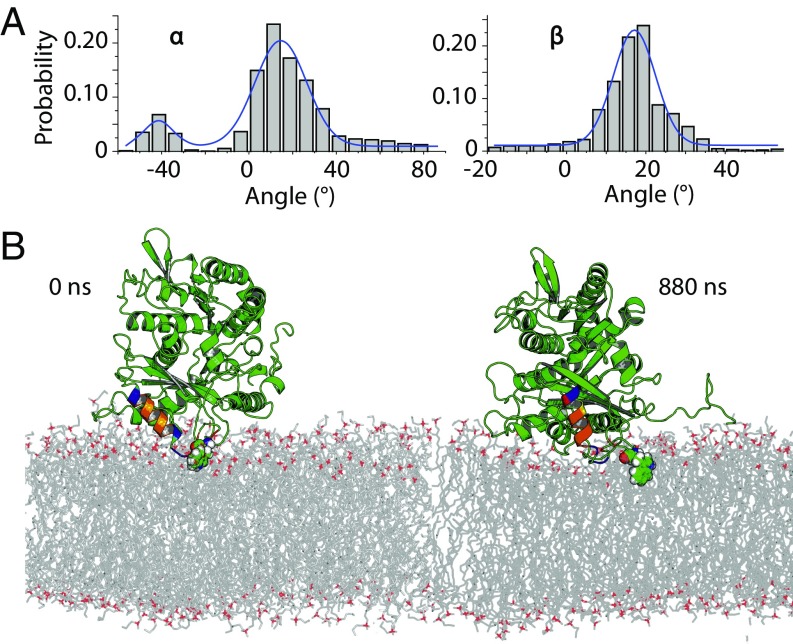
Fig. 8.
Rotational dynamics for stably bound α-tubulin on DOPE membranes from ANTON MD simulations. (A) Probability distribution of Euler rotation angles for α-tubulin. (B) Time evolution of the stable α-tubulin tethering on the DOPE membrane surfaces from all-atom MD simulations starting from the most abundant cluster in MARTINI simulations and its subsequent evolution over 880 ns of all-atom MD simulations. The insertion helix identified in MARTINI simulations is color-coded for chemical moieties as follows: blue, positively charged residues; red, negatively charged residues; orange, hydrophobic residues; and yellow, asparagine or glutamine residues. W346 is shown in molecular spheres mode. The bilayer normal and axis vector for insertion helix are shown. The unstructured C-terminal tail is shown in a ribbon mode.