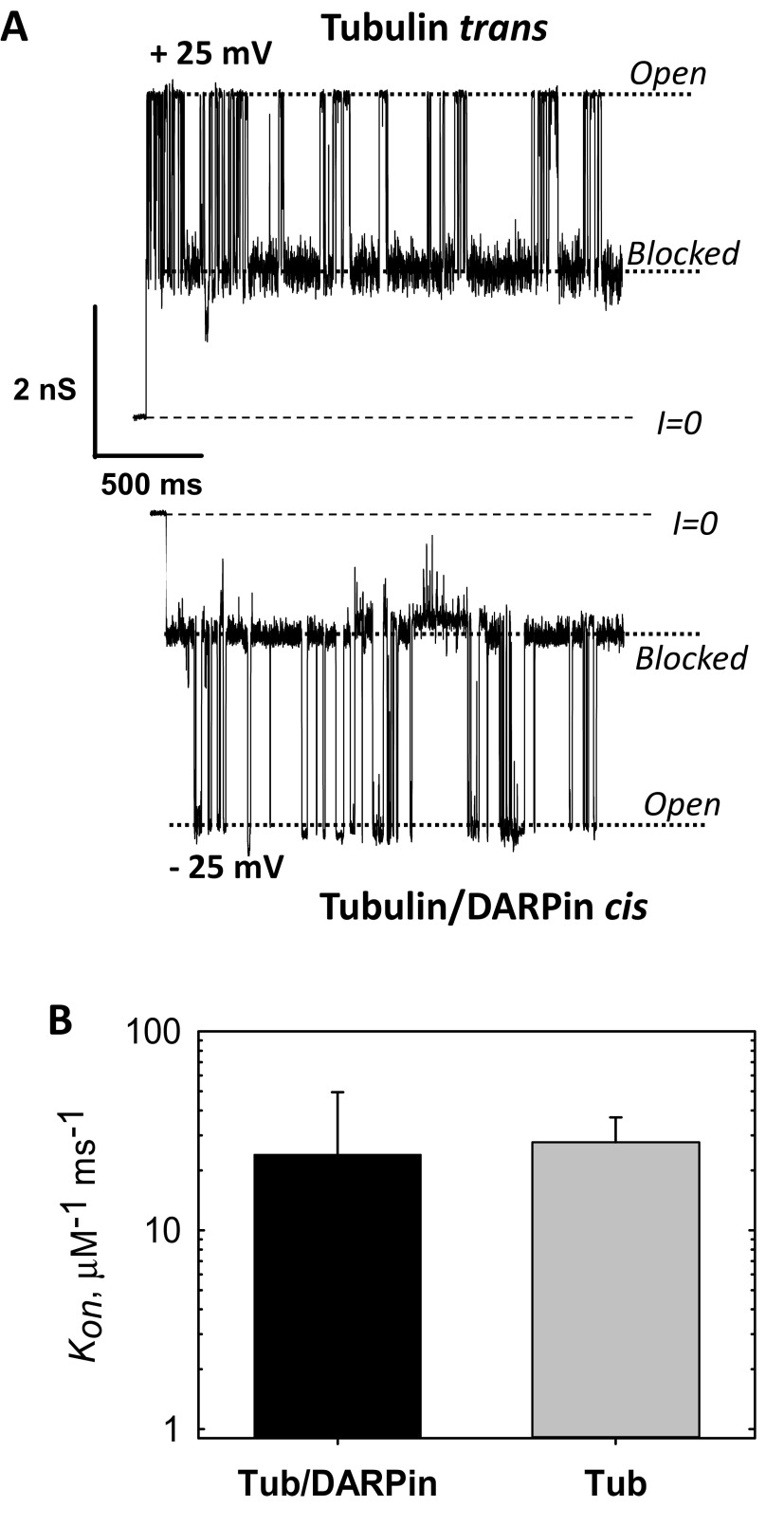
Fig. S9.
Tubulin–DARPin complex (50) does not induce less blockage of VDAC than free tubulin. (A) Representative current traces of the same single VDAC reconstituted into a planar membrane in the presence of 15 nM tubulin/DARPin (2:1) complex in the cis side of the membrane and 15 nM of free tubulin in the opposite, trans, side at −5 and + 25 mV of applied voltage, respectively. Tubulin induces characteristic blockages of VDAC conductance only when negative potential is applied at the side of tubulin addition. In our experimental setup negative potential is referred to as the cis side, or side of VDAC addition. Dashed lines indicate zero-current level and dotted lines indicate VDAC open and tubulin-blocked states. Current records were additionally filtered using a 1-kHz eight-pole digital Bessel filter. Medium consisted of 1 M KCl buffered with 5 mM Tris at pH 7.4. The planar membrane was formed from diphytanoylphosphatidylcholine (13). Recombinant mouse VDAC1 was isolated following the published protocol (78). Fifteen nanomolar tubulin was preincubated with 30 nM DARPin [purified using the published protocol (50)] on ice for 30 min before addition to the membrane-bathing solution. The current records were taken 75 min after addition of tubulin/DARPin or tubulin to the experimental chamber. Current measurements were performed as described previously (13). (B) The on-rate constants of tubulin–VDAC binding in the presence of tubulin/DARPin complex or free tubulin at 25 mV applied voltage. All experimental conditions are as in A. The data are mean of two experiments ± SE (error bars). On rates were obtained from kinetic analysis of channel blockage events as shown in A using ClampFit 10.5 software (Molecular Devices, LLC) and described previously (13).