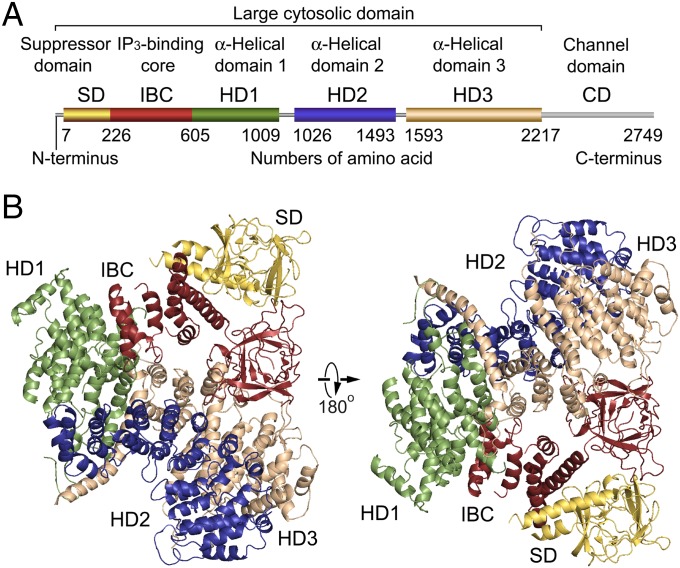
Fig. 1.
Global architecture of the IP3R large cytosolic domain. (A) Domain organization. Each domain is depicted in a different color: yellow, suppressor domain (SD; 7–225 amino acid residues); red, IP3-binding core (IBC; 226–604 residues); green, α-helical domain 1 (HD1; 605–1,009 residues); blue, α-helical domain 2 (HD2; 1,026–1,493 residues); tan, α-helical domain 3 (HD3; residues 1,593–2,217); and gray, channel domain (CD; 2,218–2,749 residues). (B) Overall crystal structure of IP3R2217 viewed from two sides. The overall arrangement of domains is consistent with four crystal structures solved using C2221 and P42 crystals (5.8–7.4 Å datasets). The left corresponds to a top view from the cytosolic side with respect to the membrane in the tetrameric IP3R (Fig. S4F).