Fig. 1.
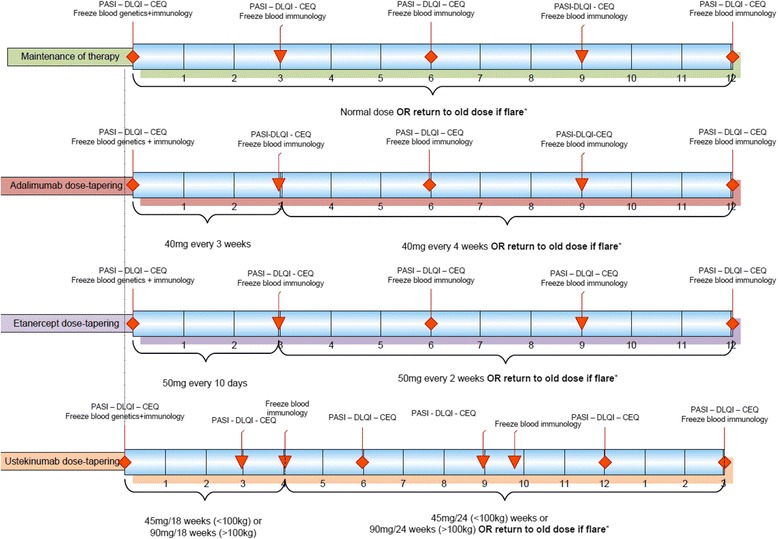
Patients using adalimumab, etanercept or ustekinumab will be randomized to dose tapering or usual care. Control visits will be planned every 3 months for the assessments of PASI, DLQI, cost-effectiveness questionnaires, drug levels and anti-drug antibodies (at trough moment). Ustekinumab blood samples for immunologic analyses are taken at deviating time points (trough moments) due to the low frequency of injections. CEQ = Cost-effectiveness questionnaires (SF-36, iMTA Medical Consumption Questionnaire and productivity cost questionnaire), PASI = Psoriasis Area and Severity Index, DLQI = Dermatology Quality of Life
