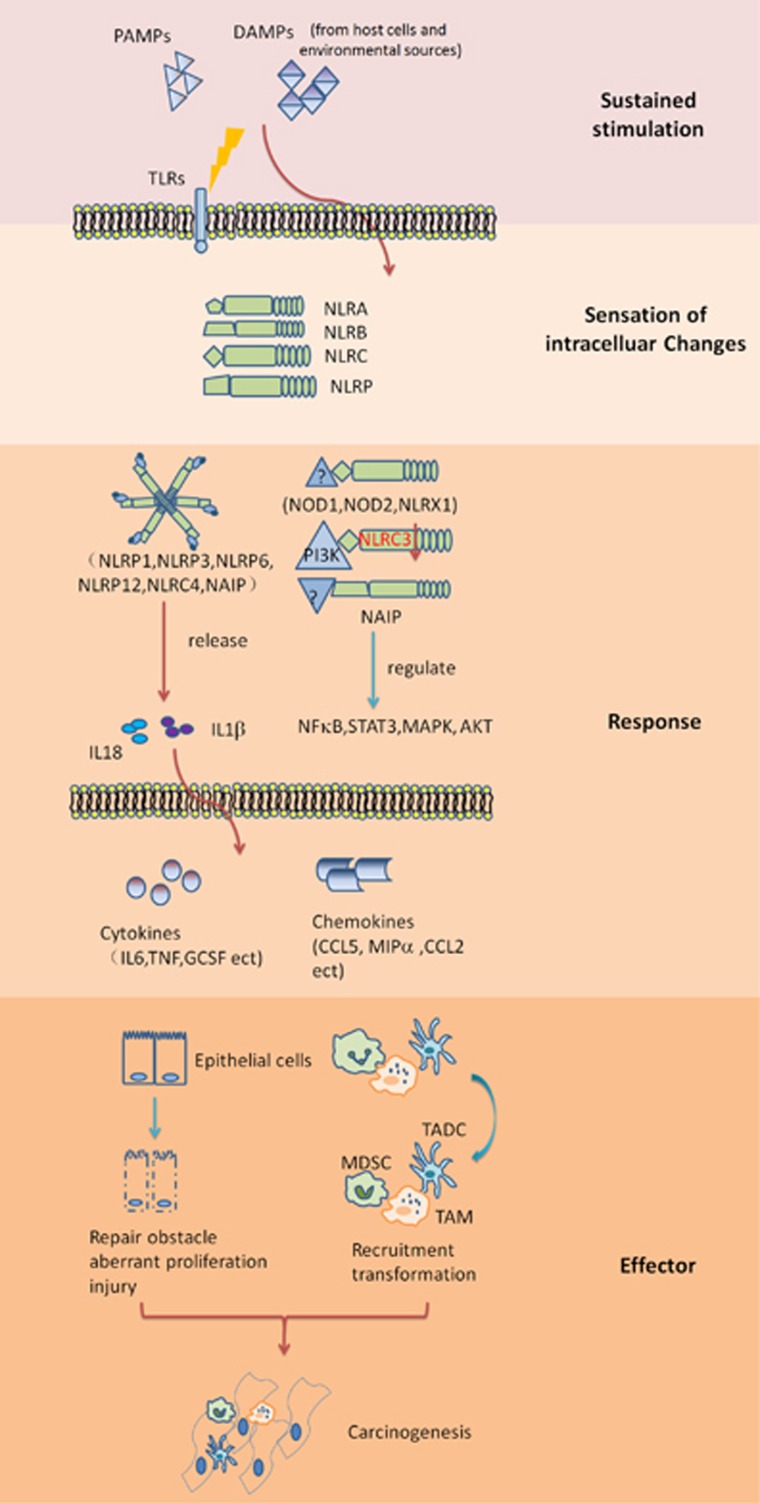
Figure 1.
Functions of NLRs in inflammation-associated carcinogenesis. Under sustained stimulation from pathogens, host cells or environmental sources, NLRs sense the intracellular changes caused by infiltrating extracellular molecules or activation of the downstream pathways of other PRRs and work to preserve cytosolic sanctity. NLRs function by associating with other proteins and through many interacting adaptors; however, details are still unclear. NLRs can form inflammasomes to release IL-18 and IL-1β, which are pro-inflammatory cytokines and related to repair and regeneration. In addition, NLRs can regulate NF-κB, STAT3, MAPK, and AKT signaling pathways in an inflammasome-independent manner. Overwhelming sustained stimulation will dysregulate the expression of NLRs, which have unknown mechanisms. Karki et al.13 recently reported that NLRC3 is downregulated in AOM/DSS-induced colorectal tumor tissue, and the ability of NLRC3 to associate with PI3K and inhibit PI3K/mTOR is impaired, which results in aberrant proliferation of intestinal epithelial cells. Dysregulated NLRs will boost inflammation by promoting the production of pro-inflammatory cytokines and chemokines and then influence the function of epithelial cells and immune cells, which favors a pro-tumor environment. DAMP, danger-associated molecular pattern; IL-18, interleukin-18; MDSC, myeloid-derived suppressing cell; NLR, nucleotide-binding and oligomerization domain-like receptor; PAMP, pathogen-associated molecular pattern; PRR, pattern recognition receptor; TADC, tumor-associated dendritic cell; TAM, tumor-associated macrophage; TLR, Toll-like receptor.