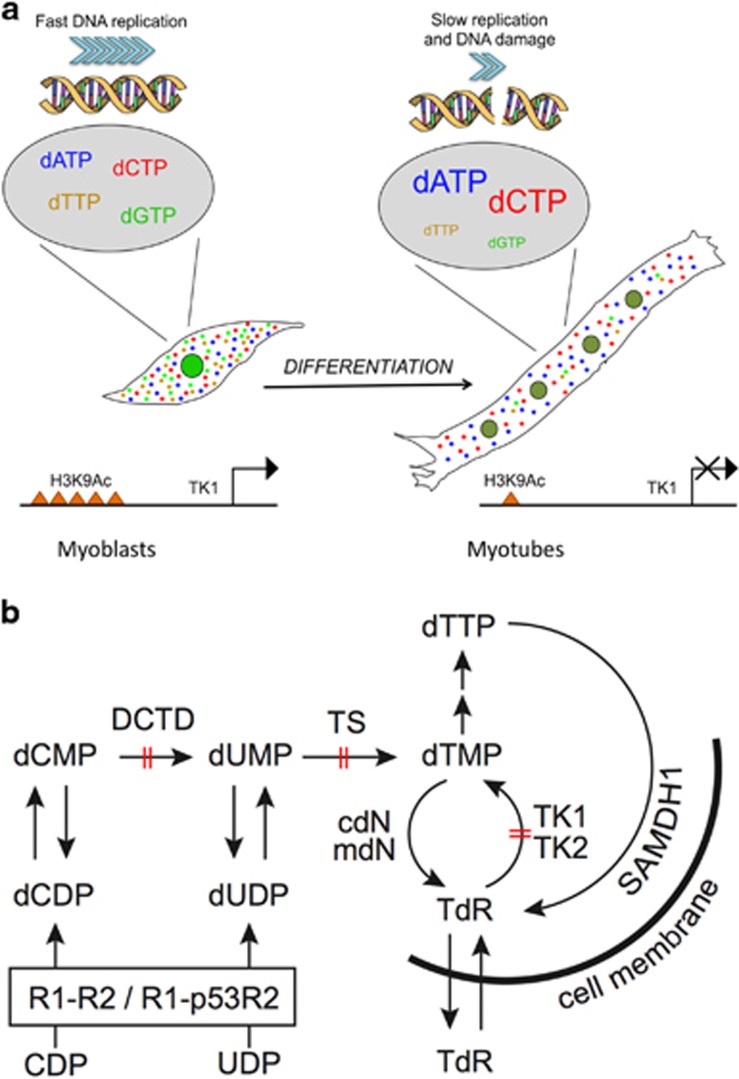
Figure 8.
Graphic summary. (a) Graphical representation of the main results of this work. The nucleotide pool is adequate and balanced in myoblasts, allowing fast DNA replication. Upon differentiation into myotubes, muscle cells deeply silence transcription of a few genes involved in dTTP synthesis, exemplified by TK1. Accordingly, such genes display markedly reduced histone H3 lysine 9 acetylation and their products are depleted in myotubes, in spite of cell cycle re-entry. Loss of key metabolic enzymes leads to a dramatic fall in dTTP and, indirectly, dGTP levels. As a consequence, cell cycle reactivation in myotubes is marred by slow DNA replication, accompanied by DNA damage. (b) Synthesis of thymidine triphosphate in mammalian cells. dTTP synthesis occurs through two pathways: de novo synthesis by RNR and salvage of TdR. The latter is initiated by two thymidine kinases, cytosolic TK1, expressed in S phase, and mitochondrial TK2, which is a constitutively expressed enzyme. During S phase, RNR is composed by homodimers of the R1 and R2 subunits, whereas in non-dividing or differentiated cells R2 is replaced by p53R2. De novo-produced thymidine monophosphate (dTMP) derives from deoxyuridine monophosphate (dUMP) via methylation by TS. In its turn, dUMP derives from (i) dephosphorylation of dUDP produced by RNR and (ii) deamination of deoxycytidine monophosphate (dCMP) by DCTD. The latter process prevails in mammalian cells.41 The salvage pathway receives TdR from the intracellular catabolism of dTMP by 5′-nucleotidases (cytosolic cdN and mitochondrial mdN) and of dTTP by the nuclear dNTP-triphosphohydrolase SAMHD1 or via uptake from the extracellular environment. In terminally differentiated myotubes DCTD, TS and TK1 are strongly repressed (barred arrows) and reactivation by p21 and p27 silencing fails to induce them to levels approaching those of proliferating cells. Thus both pathways converging on dTMP, an essential precursor of dTTP, are severely impaired in reactivated myotubes