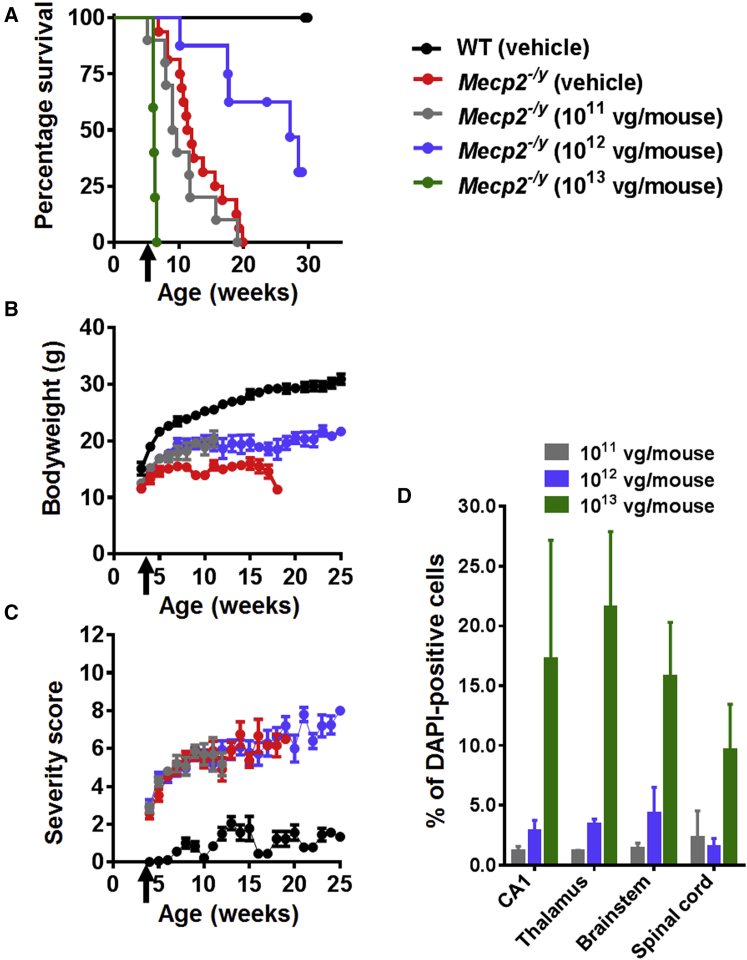
Figure 1.
Systemic Delivery of the First-Generation Vector to Mecp2−/y Mice Revealed Therapeutic Efficacy and a Narrow Therapeutic Window
(A) Kaplan-Meier survival plot for Mecp2−/y mice injected with different doses (1 × 1011 [n = 10], 1 × 1012 [n = 8], and 1 × 1013 [n = 5] vg per mouse] of first-generation vector compared to vehicle-treated animals (WT; n = 9, Mecp2−/y; n = 16). The median survival period in Mecp2−/y mice treated with 1 × 1012 vg per mouse was significantly higher than that in vehicle-treated controls (27.14 versus 11.64 weeks; p = 0.001, Mantel-Cox test). (B and C) Plots showing mean (B) body weight and (C) aggregate severity scores for Mecp2−/y mice treated with 1 × 1011 and 1 × 1012 vg per mouse or vehicle. Arrows indicate age at injection; data are presented as mean ± SEM. (D) Dose-dependent transduction efficiency (Myc-positive nuclei as a proportion of DAPI-positive nuclei) across different brain regions. Data are presented as mean ± SEM (n = 3 mice per group). CA1 indicates hippocampal region CA1.