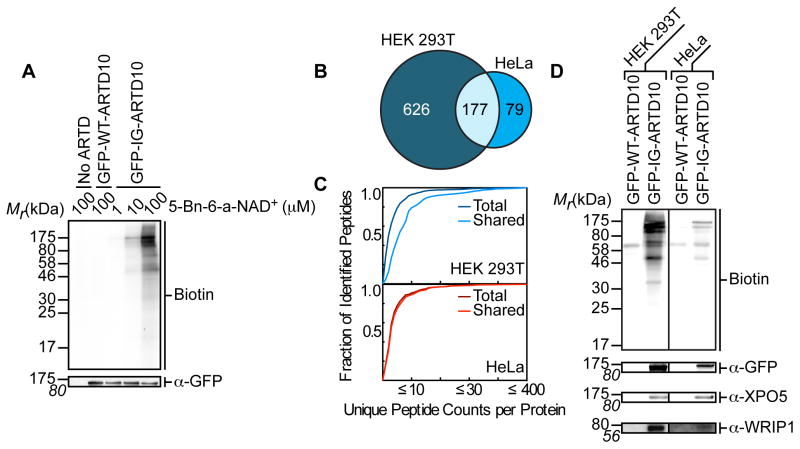
Figure 2. IG-ARTD10 Orthogonally Labels Protein Targets in the Presence of 5-Bn-6-a-NAD+.
(A) Lysate labeling by WT-ARTD10 and IG-ARTD10 in the presence of 5-Bn-6-a-NAD+. HEK 293T cells were transfected with either WT-ARTD10 or IG-ARTD10 and the resulting lysate was incubated for 2 hours in the presence of varying amounts of 5-Bn-6-a-NAD+. MARylation of direct protein targets was observed using streptavidin-HRP (Biotin). The faint bands in the WT-ARTD10 lane correspond to endogenous biotinylated proteins. Expression of ARTD10 was confirmed via immunoblot detection of GFP. Shown is a representative image from duplicate measurements.
(B) Venn diagram comparing the IG-ARTD10 targets identified via single LC-MS/MS runs in either HEK 293T or HeLa cells.
(C) Observed distribution functions for the IG-ARTD10 targets identified via single LC-MS/MS runs in either HEK 293T (top) or HeLa (bottom) cells. The distributions for the total protein pool (total) as well as the subset of proteins that were identified in both HEK 293T and HeLa (shared) are indicated. The shared targets identified in HEK 293T cells display significantly elevated peptide counts per identified protein as compared to the total target pool (p < 0.05, non-parametric Mann-Whitney U test). The shared targets identified in HeLa cells also display elevated peptide counts per protein, but the difference compared to the total target pool is not significant.
(D) Immunoblot detection of the LC-MS/MS identified ARTD10 targets (GFP-ARTD10, XPO5, WRIP1) following NeutrAvidin enrichment. MARylation levels were determined using streptavidin-HRP (Biotin). Differences in labeling efficiency between HEK 293T and HeLa lysate required separate immunoblot exposures.