Table 1. Three‐drug combinations (one targeted agent with two cytotoxic agents) reported over 4 yearsa.
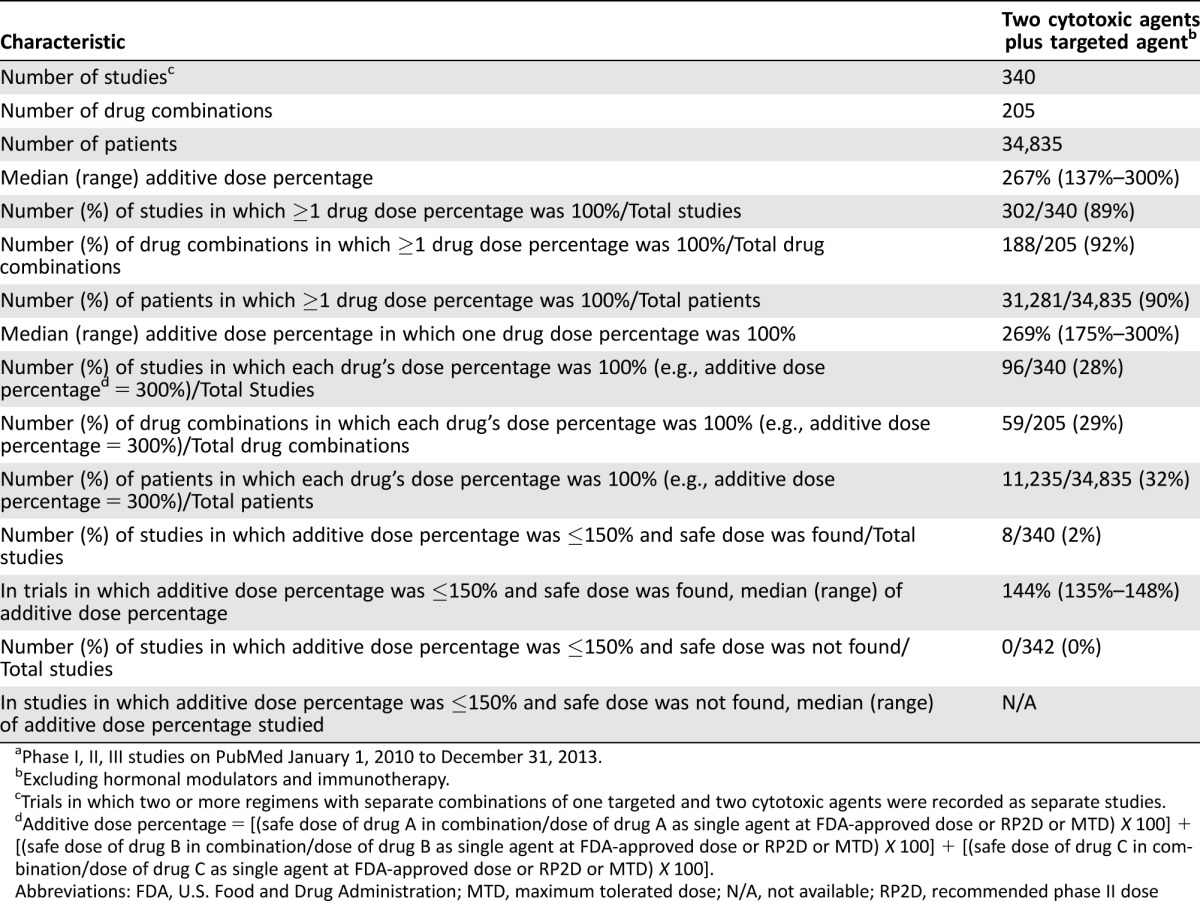
Phase I, II, III studies on PubMed January 1, 2010 to December 31, 2013.
Excluding hormonal modulators and immunotherapy.
Trials in which two or more regimens with separate combinations of one targeted and two cytotoxic agents were recorded as separate studies.
Additive dose percentage = [(safe dose of drug A in combination/dose of drug A as single agent at FDA‐approved dose or RP2D or MTD) X 100] + [(safe dose of drug B in combination/dose of drug B as single agent at FDA‐approved dose or RP2D or MTD) X 100] + [(safe dose of drug C in combination/dose of drug C as single agent at FDA‐approved dose or RP2D or MTD) X 100].
Abbreviations: FDA, U.S. Food and Drug Administration; MTD, maximum tolerated dose; N/A, not available; RP2D, recommended phase II dose
