Table 2. Summary of three drugs (one targeted and two cytotoxics) in combinationa.
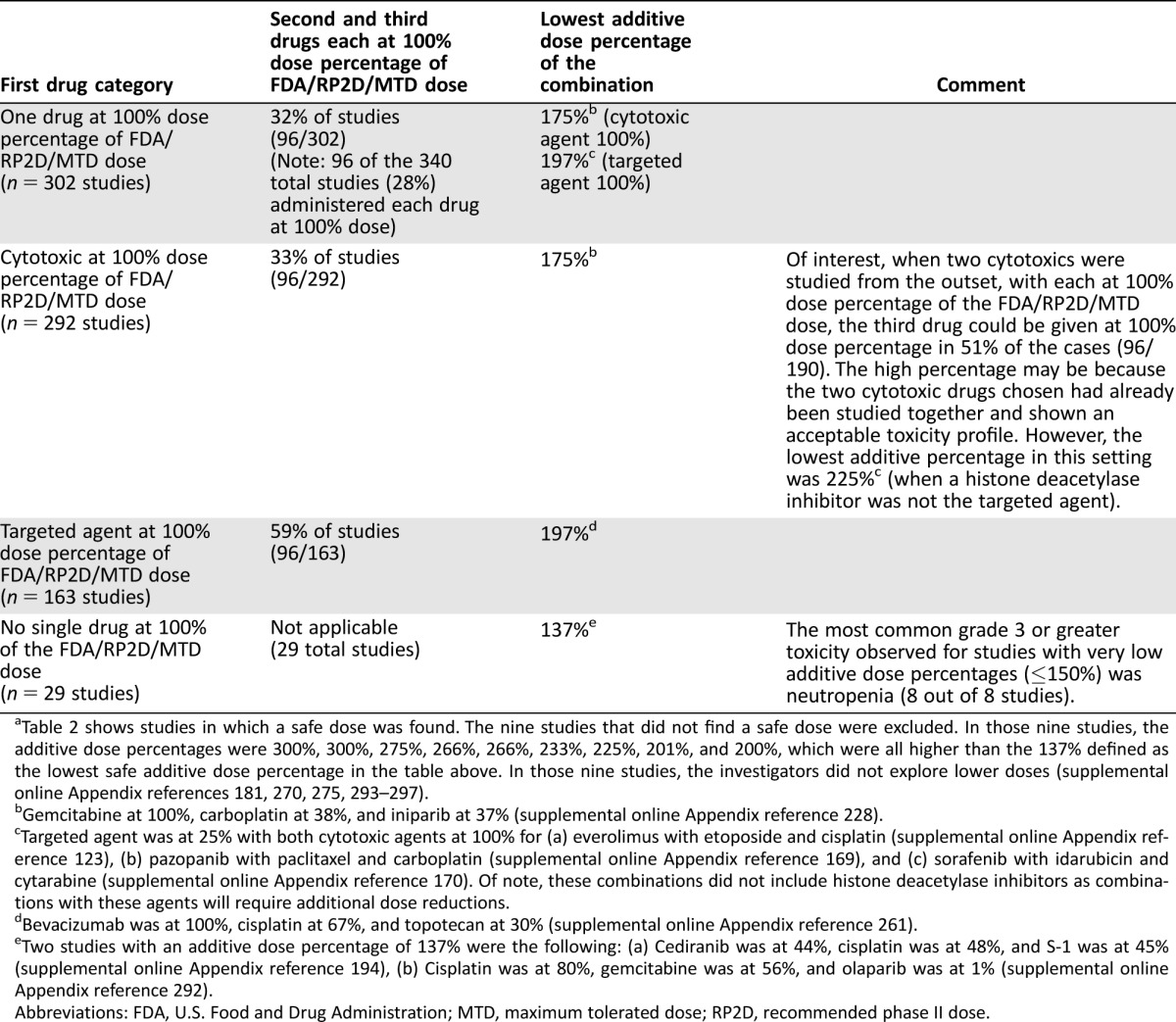
Table 2 shows studies in which a safe dose was found. The nine studies that did not find a safe dose were excluded. In those nine studies, the additive dose percentages were 300%, 300%, 275%, 266%, 266%, 233%, 225%, 201%, and 200%, which were all higher than the 137% defined as the lowest safe additive dose percentage in the table above. In those nine studies, the investigators did not explore lower doses (supplemental online Appendix references 181, 270, 275, 293–297).
Gemcitabine at 100%, carboplatin at 38%, and iniparib at 37% (supplemental online Appendix reference 228).
Targeted agent was at 25% with both cytotoxic agents at 100% for (a) everolimus with etoposide and cisplatin (supplemental online Appendix reference 123), (b) pazopanib with paclitaxel and carboplatin (supplemental online Appendix reference 169), and (c) sorafenib with idarubicin and cytarabine (supplemental online Appendix reference 170). Of note, these combinations did not include histone deacetylase inhibitors as combinations with these agents will require additional dose reductions.
Bevacizumab was at 100%, cisplatin at 67%, and topotecan at 30% (supplemental online Appendix reference 261).
Two studies with an additive dose percentage of 137% were the following: (a) Cediranib was at 44%, cisplatin was at 48%, and S‐1 was at 45% (supplemental online Appendix reference 194), (b) Cisplatin was at 80%, gemcitabine was at 56%, and olaparib was at 1% (supplemental online Appendix reference 292).
Abbreviations: FDA, U.S. Food and Drug Administration; MTD, maximum tolerated dose; RP2D, recommended phase II dose.
