Table 3. Three‐drug combinations (two targeted agents with one cytotoxic agent) reported over 4 yearsa.
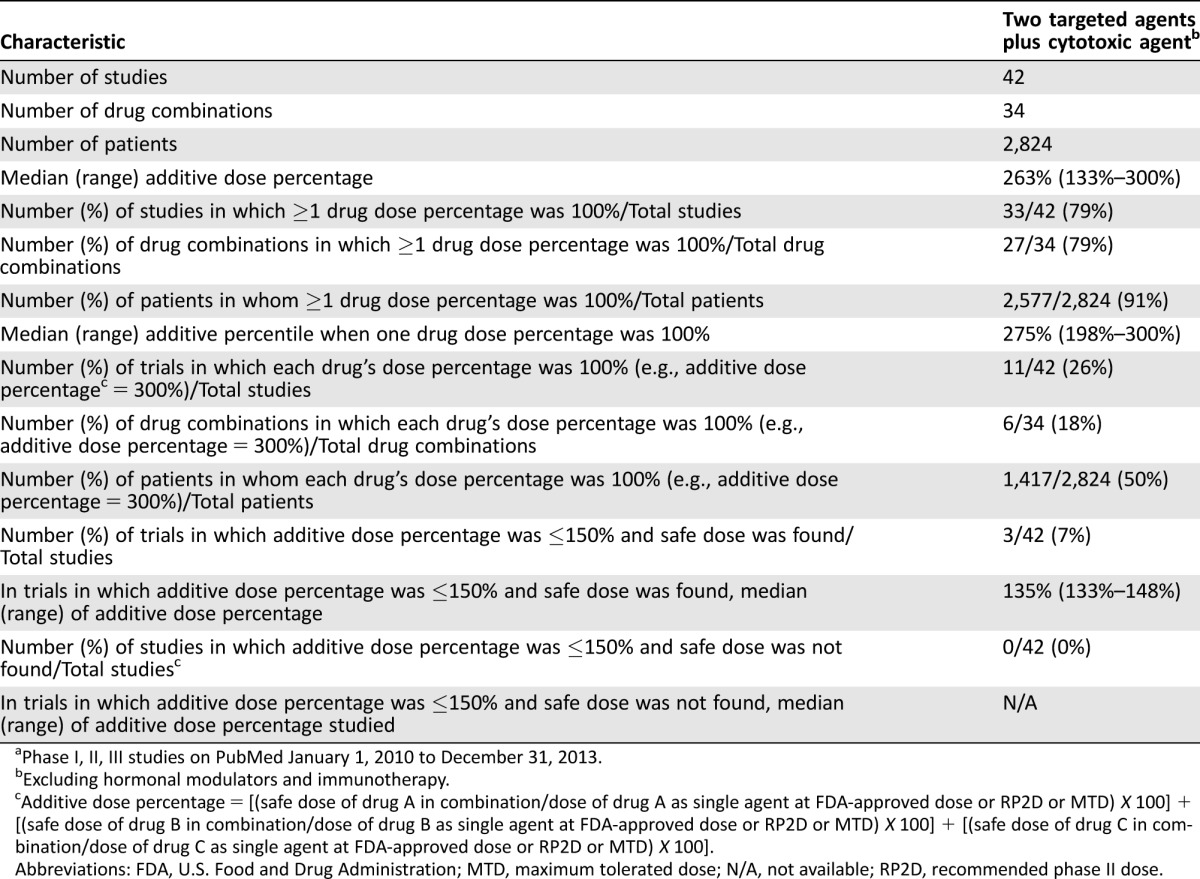
Phase I, II, III studies on PubMed January 1, 2010 to December 31, 2013.
Excluding hormonal modulators and immunotherapy.
Additive dose percentage = [(safe dose of drug A in combination/dose of drug A as single agent at FDA‐approved dose or RP2D or MTD) X 100] + [(safe dose of drug B in combination/dose of drug B as single agent at FDA‐approved dose or RP2D or MTD) X 100] + [(safe dose of drug C in combination/dose of drug C as single agent at FDA‐approved dose or RP2D or MTD) X 100].
Abbreviations: FDA, U.S. Food and Drug Administration; MTD, maximum tolerated dose; N/A, not available; RP2D, recommended phase II dose.
