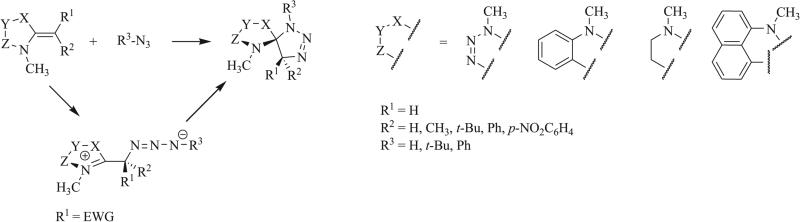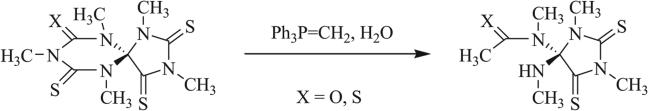Abstract
Natural products bearing a triazaspirocyclic motif have received significant attention in recent years. These compounds, which feature three nitrogen atoms attached to one quaternary carbon forming a spirocyclic scaffold, exhibit a wide range of biological activity and have promising applications in materials as well as in drug discovery. In this review article, we will discuss triazaspirocycles in Nature, their biological activity, and applications. Methods for the synthesis of triazaspirocycles as well as the reactivity of triazaspirocyclic scaffolds will be reviewed.
Keywords: 1,3 Dipolar cycloaddition; cycloreversion; N-heterocyclic carbene; nitrogen heterocycles; spirocycles; triaza
1. INTRODUCTION
Spirocyclic molecules are defined as polycyclic compounds in which two or more rings are connected by a single quaternary center designated as the spiroatom. These unique structures are widely present in natural products and find numerous applications in drug discovery due to their rigid three dimensionality. While spirocyclic systems containing all carbon quaternary centers have been the subject of numerous total syntheses and synthetic methodologies [1-3], spirocyclic compounds containing heteroatoms have historically received less attention. Of these heterocyclic spirocycles, spiroketals have been most well-studied due to their presence in insect pheromones and other natural products [4-8].
Triazaspirocycles, compounds that contain three nitrogen atoms attached to one quaternary carbon forming a polycyclic system, comprise a group of previously overlooked scaffolds that have garnered attention in recent years [9-17]. This review will describe both triazaspirocycles and their related derivatives, natural products bearing this motif and the biological activity thereof, and reactivity and synthetic routes to access triazaspiro functionalities developed to date.
2. NOMENCLATURE
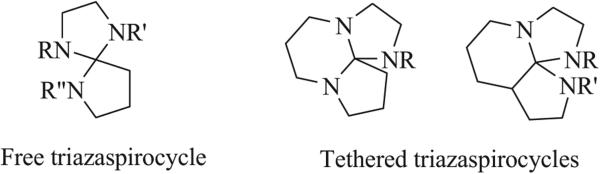
For the purposes of this review, a triazaspirocycle is defined as a polycyclic system connected by a quaternary carbon bearing three nitrogen atoms and one carbon atom. Triazaspirocycles can be categorized as one of two types; a “free” triazaspirocycle, in which the spiroatom is the only direct union between the two rings of the spirocyclic scaffold, and a “non-free” or “tethered” triazaspirocycle, in which two rings connected by a single spiroatom are also connected by another ring, Fig. (1).
Fig. (1).
Triazaspirocyclic scaffolds.
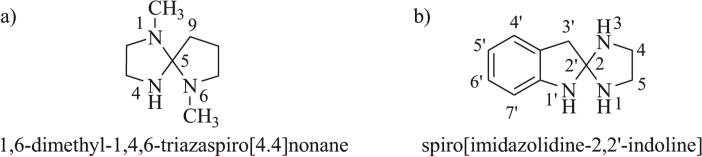
Nomenclature for triazaspirocyclic systems is decidedly complex and will thus be briefly discussed. Free triazaspiro-cycles in this review will be named according to IUPAC nomenclature [18]. Triazaspirocycles bearing only monocyclic ring systems are named by placing the prefix “spiro” in front of the name which corresponds to an acyclic hydrocarbon chain possessing the same number of atoms as the spiro-cycle (Fig. 2a). The number of atoms in each ring of the spirocycle (not including the spiroatom) is denoted in the bracketed von Baeyer descriptor (e.g. [4.4]). Numbering for each ring starts at the atom adjacent to the spiroatom. Skeletal replacement nomenclature is used to indicate heteroatoms in spirocyclic systems containing only monocyclic rings (e.g. 1,4,6-triaza). Spirocycles containing polycyclic ring components which do not conform to skeletal replacement nomenclature are denoted by indicating the position of the spiroatom on each ring between the names of the two rings (Fig. 2b). Substituents are indicated at the beginning of the name, if applicable.
Fig. (2).
a) Free triazaspirocycle with monocyclic rings; b) Free triazaspirocycle with polycyclic rings.
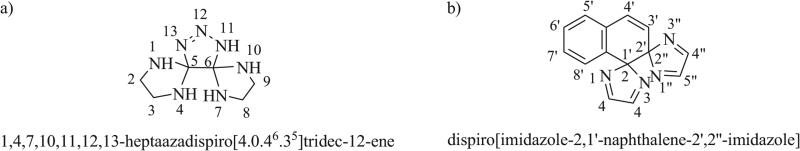
Polyspiro ring systems follow similar rules as monospiro systems (Fig. 3a). The number of spiro unions is denoted by “dispiro,” “trispiro,” etc. Numbering begins at the smallest ring at the atom adjacent to the spiroatom and continues through each spiroatom to the terminal ring system before returning back to the first spiro atom, taking the shortest pathway through each spiroatom. The number of atoms linking each spirocycle is denoted in the bracketed von Baeyer descriptor, using superscripts to indicate the locant of spiroatoms reached for a second time (e.g. [4.0.46.35]). Skeletal replacement nomenclature is used to indicate heteroatoms in polyspirocyclic systems containing only monocyclic rings.
Fig. (3).
a) Free polyspiro triazaspirocycle with monocyclic rings; b) Free polyspiro triazaspirocycle with polycyclic rings.
Polyspiro ring systems containing polycyclic ring components which do not conform to skeletal replacement nomenclature are denoted by indicating the position of the spiroatom on each ring between the names of the rings (Fig. 3b).
Tethered triazaspirocycles bearing two ortho fusions are named using nomenclature for fused ring systems (Fig. 4a). Tethered triazaspirocycles containing bridges as well as fused ring systems are named according to both fused ring system nomenclature and the von Baeyer system for naming polycyclic compounds (Fig. 4b). Bivalent bridges derive their names from the corresponding unbranched hydrocarbon containing the same number of atoms, changing the final letter to an “o” instead of an “e” (e.g. methano). The locants of the attachment points of the bridge are noted before each bridge name. Bivalent homogeneous heteroatom bridges derive their names from the traditional substitutive prefix and are distinguished using the prefix “epi” (e.g. epimino). Composite bridges are named by linking the names of the two simple bridges together, omitting any “epi” prefixes (e.g. azenometheno). The locants of composite bridges are written in the order denoted by the bridge name. A bivalent monocyclic bridge is named using the prefix “epi” and the prefix traditionally used to describe fused ring systems (e.g. epicyclopenta).
Fig. (4).
a) Tethered triazaspirocycle; b) Tethered triazaspirocycle with bivalent bridge.
3. THREE DIMENSIONALITY
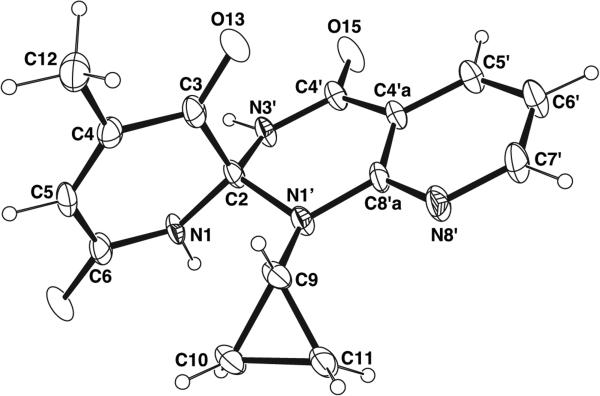
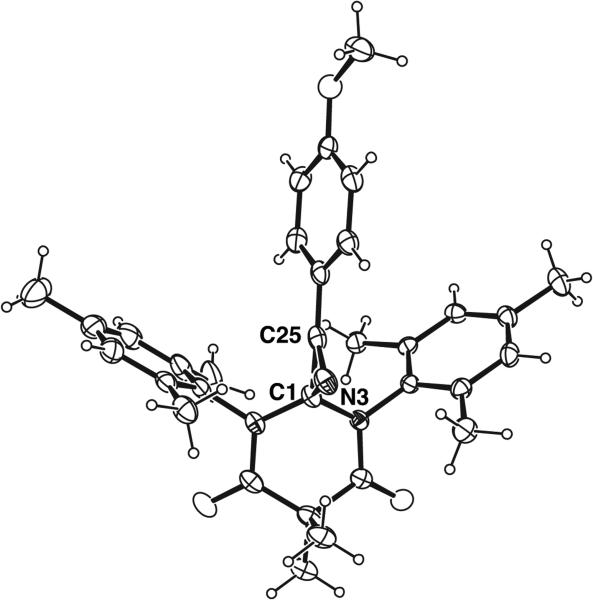
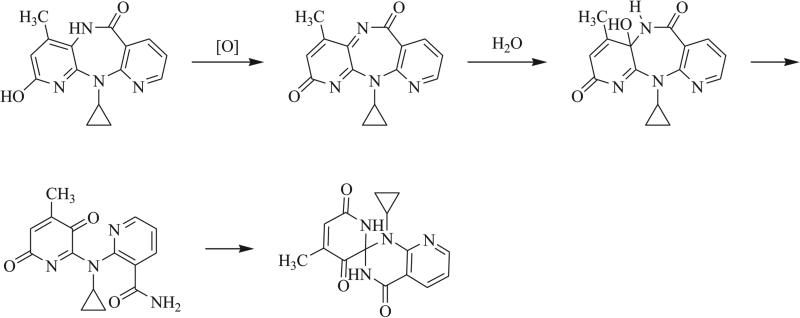
Triazaspirocycles afford unique three dimensional scaffolds in both free and tethered conformations. Rigid three dimensional structures are of the utmost importance in medicinal chemistry as strained spirocyclic systems exhibit a reduced conformational entropy penalty upon binding to a protein or other drug target. In particular, spiro unions located in the center of a molecule provide a more rigidifying effect than peripheral spiro unions. The three-dimensionality of triazaspirocycles is evident in their crystal structures. In an example of a triazaspirocyclic compound synthesized following the oxidation of 2-hydroxynevirapine, the X-ray crystal structure of the triazaspiro compound displays a nearly tetrahedral geometry at the spiro atom (Fig. 5) [19].
Fig. (5).
X-ray crystal structure of 1'-cyclopropyl-4-methyl-spiro[pyridine-2,2'-pyrido[2,3-d]pyrimidine]-3,4',6-trione [19].
Free triazaspirocycles bearing smaller rings begin to experience ring strain and deviate from tetrahedral geometry. In an azirine triazaspirocycle synthesized by cycloaddition of an N-heterocyclic carbene (NHC) with 4-methoxybenzonitrile, the reduced length of the C25-N3 bond (1.2753 Å) results in a reduction of the C25-C1-N3 bond angle as compared to the corresponding aziridine, which typically exhibits uniform 60 degree bond angles, Fig. (6) [20]. The N3-C25-C1 and the C25-N3-C1 bond angle were found to increase to 67.81 and 64.32 degrees, respectively.
Fig. (6).
X-ray crystal structure of 4,8-dimesityl-2-(4-methoxyphenyl)-6,6-dimethyl-1,4,8-triazaspiro[2.5]oct-1-ene-5,7-dione [20].
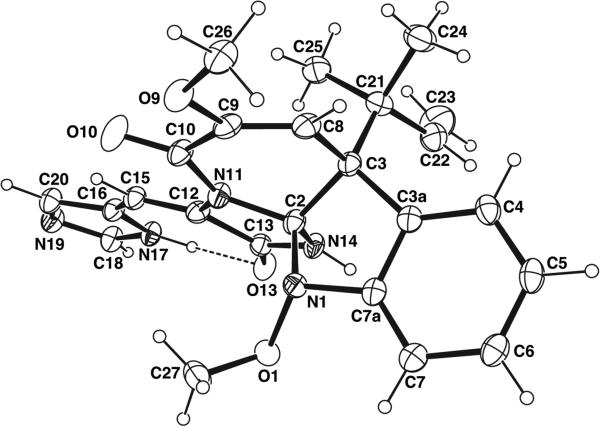
Crystal structures of tethered triazaspirocycles show significant degrees of ring distortion and lead to unique structures. A recent crystal structure of the indole alkaloid oxaline shows an envelope conformation for the indole five membered ring as well as an intramolecular N-H---O hydrogen bond which results in a low dihedral angle for the two C3N2 rings, Fig. (7) [21]. In the crystal structure of dibromoagelaspongin, an envelope conformation is observed for both the tetrahydroimidazolone and the imidazole rings, while the pipe-ridine ring C exhibits a distorted twist-conformation [22].
Fig. (7).
X-ray crystal structure of oxaline, with hydrogen bonding shown as a dashed line [21].
4. NATURAL PRODUCTS AND BIOACTIVITY
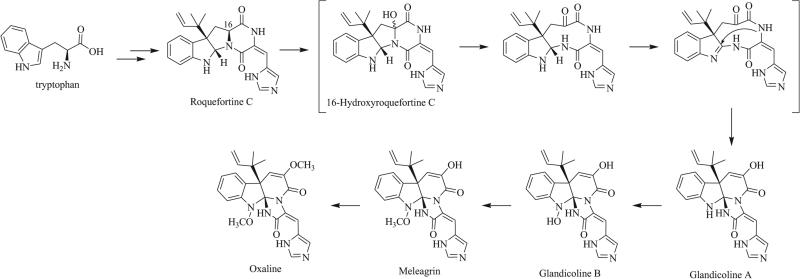
A number of natural products contain the triazaspiro motif, most commonly in the form of a tethered triazaspirocycle. The majority of naturally derived triazaspirocycles are of Penicillium origin, and of those, most have been shown to originate from biosynthetic transformations of roquefortine C [9, 12, 13, 23-25]. The biosynthesis of triazaspirocycles of Penicillium origin has been a subject of interest since the 1980s (Scheme 1). In a radiolabeling study, Vleggaar observed the incorporation of radiolabeled tryptophan into both roquefortine C and oxaline, providing the first evidence to suggest a biosynthetic pathway containing indole alkaloids roquefortine C, glandicolines A and B, meleagrin (also called meleagrin A), and oxaline [23].
Scheme 1.
Proposed biosynthesis of triazaspirocycles glandicoline A, glandicoline B, meleagrin, and oxaline [22].
Vleggaar hypothesized the pathway to begin with the hydroxylation of roquefortine C, rearrangement to give glandicolines A and B, and methylation to provide meleagrin and oxaline. It was unclear from this study, however, whether oxaline was made biosynthetically via a direct oxidation and rearrangement of roquefortine C, as is suggested by the proposed biosynthetic pathway, or if it was instead made via a separate pathway. Further radiolabeling studies by Kozlovski provided evidence of direct oxidation and rearrangement as incorporation of exogenous 14C-labeled roquefortine C into the proposed downstream metabolites was shown to occur in growing cultures of Penicillium glandicola [24].
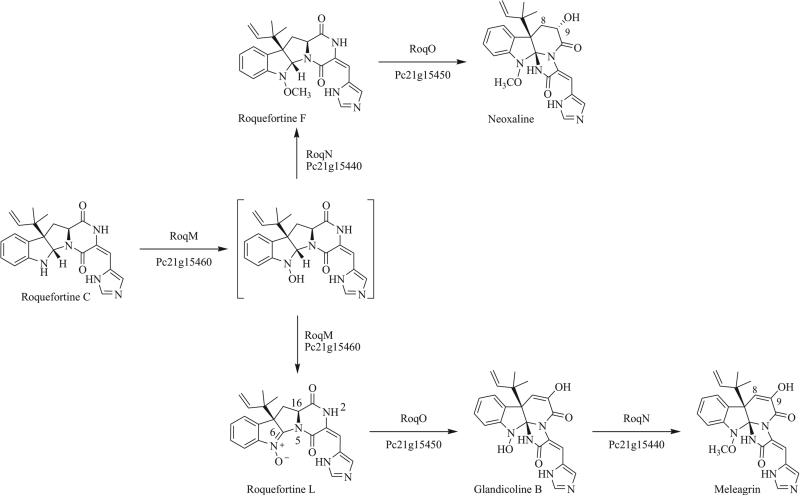
In 2008, the genome of Penicillium chrysogenum was sequenced [26], which led Martín et al. to discover a cluster of genes encoding the biosynthesis of roquefortine C and related triazaspirocyclic alkaloids. Through a series of RNA interference (RNAi) gene silencing studies, they reinforced the previously proposed biosynthesis by assigning genes to each individual step of the biosynthesis [9]. However, upon elaboration of this study using a more highly expressing strain and obtaining structural characterization for each of the observed metabolites, Vreeken reported several new metabolites whose biosyn-thesis was encoded by the roquefortine C gene cluster (Scheme 2). Among the new metabolites reported for the first time was neoxaline, an 8,9-dehydro analog of meleagrin initially isolated from cultures of Aspergillus japonicas [27]. Although neoxa-line had not previously been observed in cultures of Penicillium fungi, its C-9 stereoisomer epi-neoxaline has been reported in a number of species of Penicillium [28]. The presence of new metabolite roquefortine L prompted Vreeken to propose a revision to the roquefortine C biosynthetic pathway, in which triazaspirocycles were formed by nucleophilic transannular attack of a nitrone [12, 13].
Scheme 2.
Biosynthesis of roquefortine C derived triazaspirocycles [12, 13].
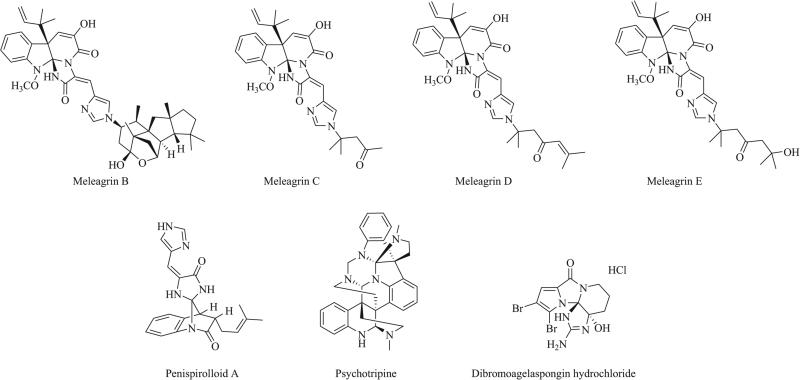
Natural products containing the triazaspiro moiety have been shown to exhibit a wide range of biological activity. Glandicoline B, first isolated from cultures of P. glandicola [29], has been shown to exhibit antimicrobial activity against Staphyloccoccus aureus, Micrococcus luteus and Escherichia coli at 100 μg/mL [30]. Meleagrin has demonstrated suppression of bacterial fatty acid synthesis through inhibition of enoyl-ACP reductase isoform FabI [11] as well as anti-settlement activity against the barnacle cyprid Banalus amphitrite through inhibition of its molting cycle and attachment mediated by adhesive plaque matrix protein (APMP) [10]. Meleagrin has also shown cytotoxic effects against a number of tumor cell lines, with IC50 values ranging from 2.73 to 12.8 μM [31]. Several imidazole-substituted biosynthetic derivatives of meleagrin have been isolated from deep sea Penicillium fungi, Fig. (8) and were shown to exhibit a range of cytotoxic effects against HL-60, MOLT-4, A-549, and BEL-7402 cell lines. Meleagrin B displayed moderate cytotoxicity, with IC50 values in the 1.8 to 6.7 μM range for each cell line. Meleagrin C displayed less potent activity, particularly against the HL-60 cell line, where IC50 values were greater than 50 μM [32]. Meleagrins D and E showed significantly weaker activity than meleagrins A-C against the A-549 cell line and displayed virtually no activity against the HL-60 cell line (IC50 > 100 μM) [33]. Neoxaline along with oxaline, a C9-OMe biosynthetic derivative of meleagrin first isolated from Penicillium oxalicum [34], has displayed anti-proliferative activity against Jurkat cells from a human T cell leukemia. In particular, oxaline was shown to arrest the cell cycle at the M phase through inhibition of tubulin polymerization [15].
Fig. (8).
Meleagrins B-E, penispirolloid A, psychotripine, and dibromoagelaspongin hydrochloride.
Another natural product containing a triazaspiro scaffold is penispirolloid A, whose structure features a free spiro union and a bridged fused ring system. Penispirolloid A was isolated from a halotolerant Penicillium species and was shown to inhibit settlement of the fouling organism Bugula neritina with an EC50 of 2.4 μg/mL [35]. Other triazaspirocyclic natural products include psychotripine, a unique compound featuring eight fused rings and three bivalent nitrogen-containing bridges isolated from the leaves of Psychotria pilifera [36], and dibromoagelaspongin hydrochlo-ride, a guanidine-derived species isolated from the marine sponge Agelas sp. [23]. Neither of these two compounds has demonstrated any biological activity to date.
5. SYNTHESIS OF TRIAZASPIROCYCLES
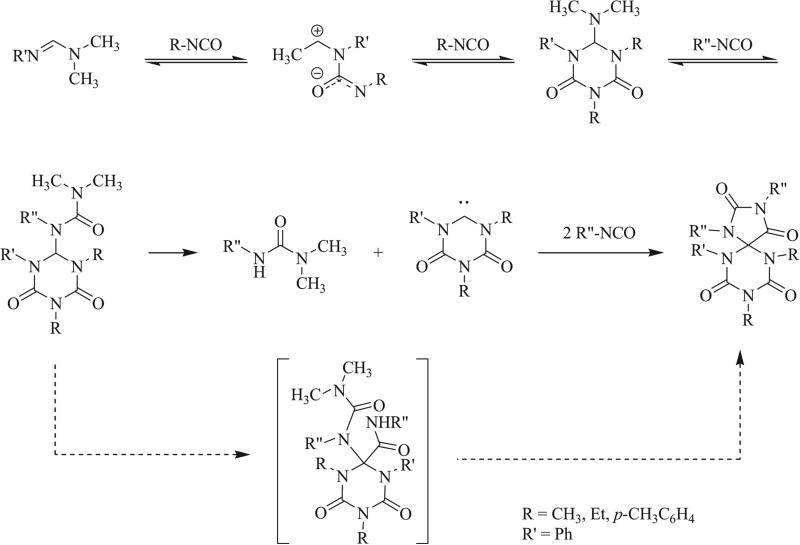
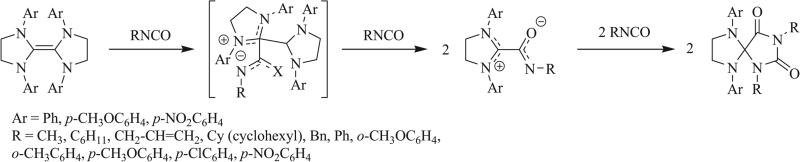
The most common synthetic method for the construction of triazaspirocycles is the 1,3 dipolar cycloaddition (1,3 DCA). In this particular reaction, a 1,3 dipole undergoes a [3+2] cycloaddition with a dipolarophile to form a five-membered ring. 1,3 Dipoles share four electrons across a π system of three atoms and typically react with dipolarophiles such as carbenes and olefins. Three publications detailing the isocyanate-based syntheses of triazaspirocycles were published within a three month span in 1968. Ulrich et al. and Dyer et al. each reported generation of a triazaspirocyclic core following the cycloaddition of two molecules of isocyanate and one molecule of di-substituted formamide/formamidine [37, 38]. Each proposed the reaction to proceed via the addition of the isocyanate to the formamide to produce a 1,4 dipole. Reaction of the 1,4 dipole with a second molecule of isocyanate would provide the triazine. Insertion of the isocyanate into the C-N(CH3)2 bond would provide the urea moiety. Dyer et al. proposed the cyclization to form the triazaspirocycle to proceed following the carbonylation of the triaza center. Ulrich and Richter later demonstrated the reaction to proceed via an NHC intermediate, which was formed following the elimination of the N,N-dimethyl urea (Scheme 3) [39].
Scheme 3.
1,3 Dipolar cycloaddition of isocyanates and N,N-dimethylformamides [37, 38].
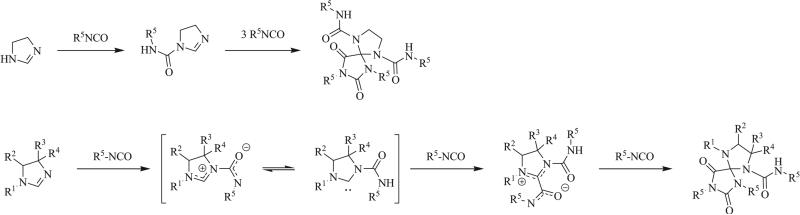
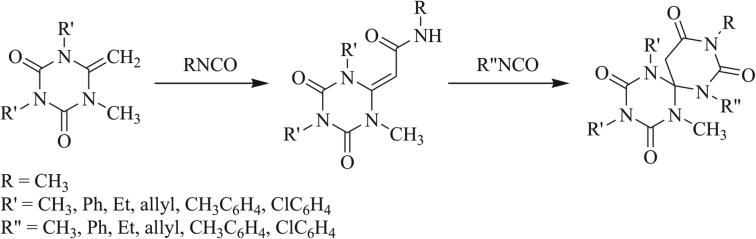
A variety of formamides and formamidines have been implemented in the synthesis of triazaspirocycles. Richter first reported the reactions of a pyrrolopyrimidine and 2-methylimino-1-methyl-pyrrolidine to give tethered triazaspirocycles (Scheme 4) [40]. Reactions of isocyanates with N-substituted imidazolidines resulted in N-urea substituted spirocycles while unsubstituted imidazolidines result in the di-substituted adducts [41-43]. Giesecke and Hocker demonstrated the scope of the former reaction, determining that imidazolidines bearing electron withdrawing substituents showed decreased reactivity [44-46].
Scheme 4.
1,3 Dipolar cycloaddition of isocyanates with unsubstituted and N-substituted imidazolidines [44].
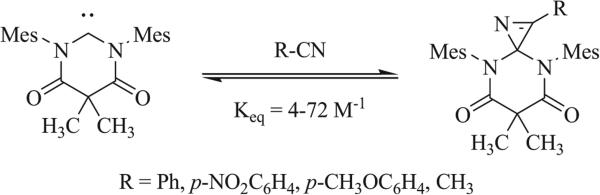
A number of NHCs have been implemented in the synthesis of triazaspirocycles, exhibiting reactivity with both aryl and vinyl isocyanates (Scheme 5) [47, 48]. Formation of NHCs can proceed via the corresponding metal-carbene complex [49], from the elimination of a haloform (CHX3) [50], or from deprotonation of an imidazolium species [51, 52]. Elimination of carbamates, analogous to the urea elimination observed by Ulrich et al. has been described as well in the synthesis of NHCs [53]. Dimerization of imidazole-based carbenes results in the synthesis of bi-imidazoylidenes [54], which, upon addition of isocyanates, have been shown to produce a 1,4 dipole intermediate similar to that observed with the addition of isocyanates to NHCs (Scheme 6) [55-58]. Bi-imidazoylidenes have also been shown to oxidize in air to form peroxides (Scheme 7). Rearrangement and loss of OH− produces the corresponding triazaspirocycle [59].
Scheme 5.
1,3 Dipolar cycloaddition of an NHC with a vinyl isocyanate [50].
Scheme 6.
Triazaspirocycle formation via isocyanate addition to bi-imidazoylidenes [55-58].
Scheme 7.
Imidazoylidene dimerization and oxygen-mediated ring expansion to triazaspirocycle [59].
Enamines can also serve as proxies for the formamidine functionality (Scheme 8). Use of enamines by Etienne et al. as reagents in isocyanate cycloaddition reactions allows for the formation of [5,5] triazaspirocycles [60, 61].
Scheme 8.
Formation of [5.5] triazaspirocyclic systems via Michael addition of isocyanates to enamines [60, 61].
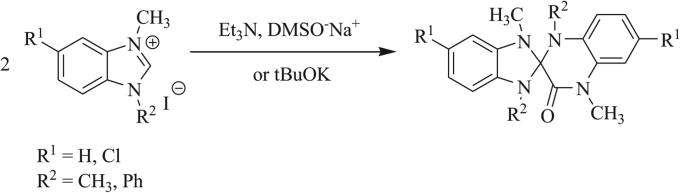
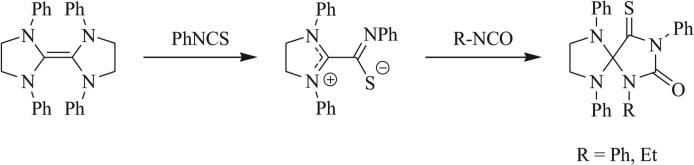
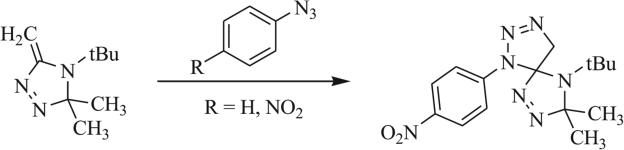
Other 1,3 dipoles such as isothiocyanates and isoselenocyanates have been similarly implemented in the synthesis of triazaspirocycles [53, 55, 56, 62]. These dipoles have been used to generate dithione/diselenone species as well as in conjugation with isocyanates to produce mixed cycloaddition products such as thioxoimidazolidinones (Scheme 9) [53, 54, 62]. Construction of triazaspirocycles is also commonly carried out with azides. Numerous examples of azide-derived triazaspirocycles have been reported using methylene-substituted heterocycles (Schemes 10 and 11) [63-67]. Nitrile imines, formed from the corresponding hydrazonoyl chlorides, have also been implemented in the synthesis of triazaspirocycles (Scheme 12) [67-69].
Scheme 9.
Synthesis of thioxoimidazolidinones via mixed 1,3 dipolar cycloaddition [62].
Scheme 10.
1,3 Dipolar cycloaddition of azides with methylene imidazolidines, methylene tetrazoles, and methylene pyrimidines [63].
Scheme 11.
1,3 Dipolar cycloaddition of azides with a methylene triazole [67].
Scheme 12.
Synthesis of a [4.5] triazaspirocycle via nitrile imine 1,3 dipolar cycloaddition [69].
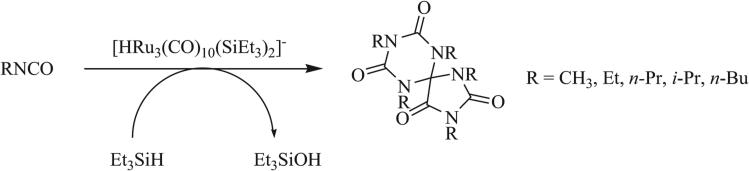
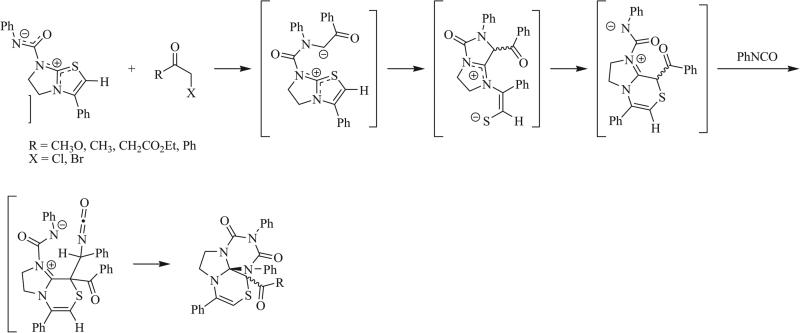
While 1,3 dipolar cycloaddition remains the most common method for the synthesis of triazaspirocycles, other cycloadditions have been reported to give this spiro adduct. Herrmann and Süss-Fink reported in 1985 the cyclization of isocyanates in the presence of a ruthenium catalyst and triethylsilane to give [4.5] spirocyclic systems with alternating CO-NR units (Scheme 13) [4]. 1,4 Dipolar cycloadditions have been observed in the case of a thiazolium-betaine, which was shown to undergo reaction with phenacyl bromide to form a 1,4 dipole (Scheme 14) [70, 71]. The C=N bond of phenyl isocyanate served as the dienophile to give the fused triazaspirocycle. The reversible [2+1] cycloaddition of nitriles and NHCs was reported by Moerdyk and Bielawski in a paper detailing the synthesis of cyclopropenes by cycloaddition of NHCs and alkynes (Scheme 15) [20].
Scheme 13.
Ruthenium-catalyzed self-cyclization of isocyanates [4].
Scheme 14.
1,4 Dipolar cycloadditions of a thiazolium-betaine with phenyl isocyanate [70, 71].
Scheme 15.
[2+1] Cycloaddition of NHCs and nitriles [20].
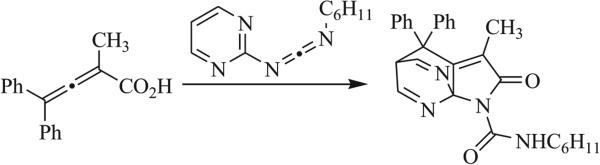
A few examples of [4+2] cycloadditions have been shown to yield triazaspirocycles. Allenic acids and pyrimidinyl diimides have been reported by Orahovats et al. to produce an (azenometheno)pyrrolo[2,3-b]pyridine triazaspirocyclic system (Scheme 16) [72]. Quast et al. also reported the [4+2] cycloaddition of an isopropylidenedihydrotetrazole with a proposed 1,3-diazabutadiene intermediate formed the tetrazole via a [1,4]-hydride shift and subsequent loss of N2 (Scheme 17) [65].
Scheme 16.
[4+2] Cycloaddition of an allenic acid and a pyrimidinyl diimide [72].
Scheme 17.
[4+2] Cycloaddition of an isopropylidenedihydrotetrazole with a 1,3-diazabutadiene intermediate [65].
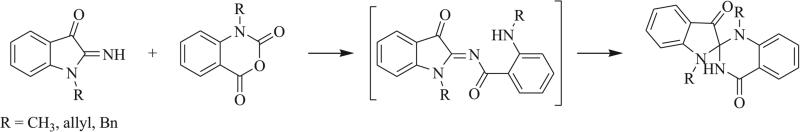
Another common approach for the synthesis of triazaspirocycles is intramolecular nucleophilic addition of nitrogen atoms to a double bond. A number of 5- and 6-exo-trig cyclizations have been reported [73-75], such as the intramolecular cyclization following the oxidation of 2-hydroxy nevirapine observed by Marques et al. (Scheme 18) [19]. Coppola et al. [76] and Oine et al. [77] also reported the synthesis of [4.5] triazaspirocycles via a 6-endo-trig cyclization (Scheme 19).
Scheme 18.
Hydrolysis, rearrangement, and 5-exo-trig cyclization of 2-hydroxy nevirapine [19].
Scheme 19.
Synthesis of spiro[indoline-2,2'-quinazoline]-3,4'(3'H)-dione via 6-endo-trig cyclization [76].
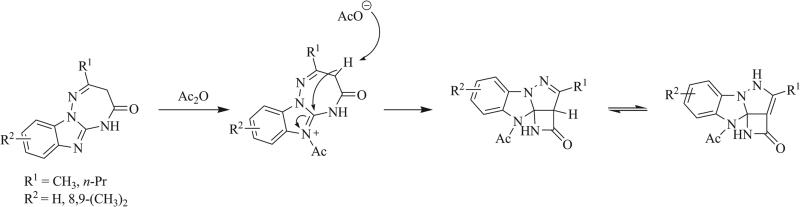
Additionally, a transannular ring contraction promoted by acetic anhydride has been shown by Avendaño et al. to produce novel triazaspirocyclic beta-lactams (Scheme 20) [78]. Diamine condensation with amides has been used to form [4.5] and [4.6] triazaspirocycles (Scheme 21) [79, 80].
Scheme 20.
Synthesis of fused beta-lactams via acetic anhydride promoted transannular rearrangements [78].
Scheme 21.
Triazaspirocycle synthesis via diamine condensation [79].
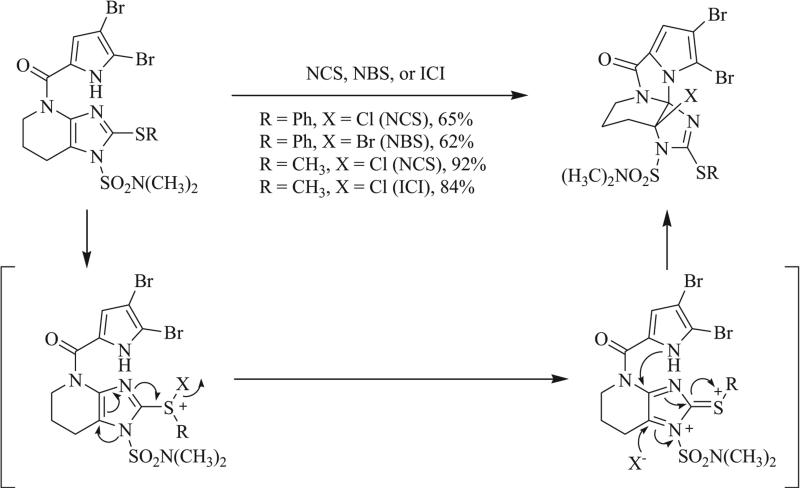
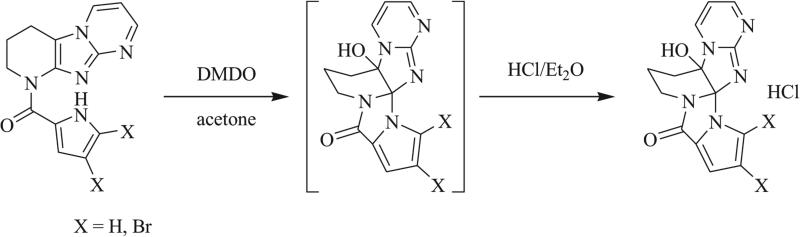
Unlike bimolecular cycloadditions, intramolecular cyclizations often proceed stereo- and regioselectively and are thus advantageous in achieving asymmetric synthesis of triazaspirocyclic systems. In the total synthesis of dibromoagelaspongin by Feldman et al., oxidative cyclization to form the tethered triazaspirocycle was shown to occur in the presence of N-bromosuccinimde (NBS) or N-chlorosuccinimide (NCS) (Scheme 22) [81-83]. Following mechanistic investigation of this reaction, Feldman et al. demonstrated this transformation to take place via a Pummerer reaction, in which electrophilic chlorination of the –SR group led to elimination to form a sulfonium intermediate, which subsequently underwent nucleophilic attack by the adjacent dibromopyrrole ring. Feldman et al. had initially synthesized the triazaspirocyclic core of dibromoagelaspongin via intra-molecular ring opening of an epoxide, which led to the triazaspirocyclic hemiaminal following acidic workup (Scheme 23) [82, 84].
Scheme 22.
Pummerer reaction to access dibromoagelaspongin core [81-83].
Scheme 23.
Synthesis of dibromoagelaspongin core via intramolecular epoxide ring opening [84].
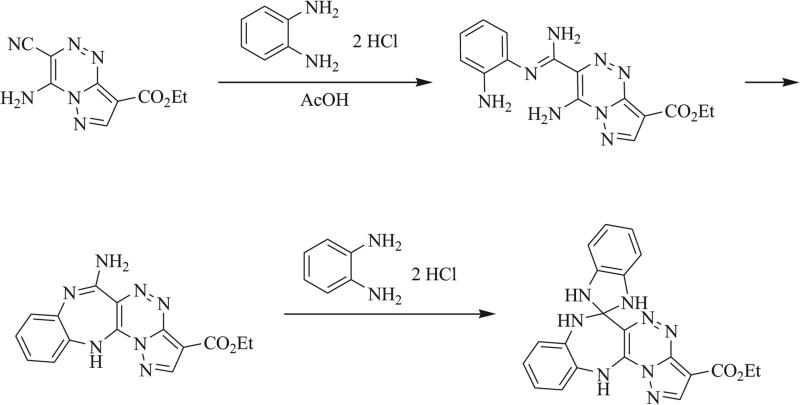
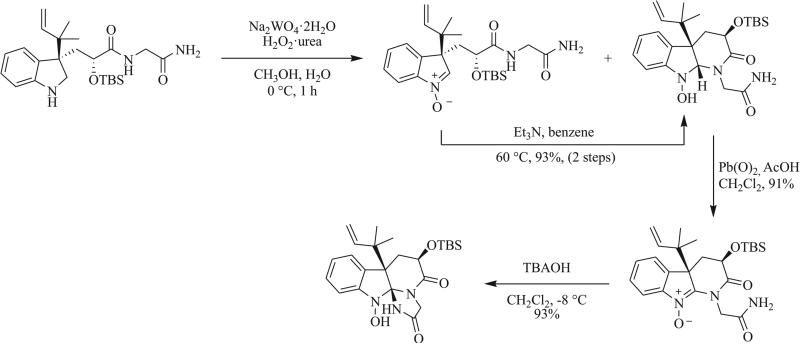
In their efforts towards the total syntheses of neoxaline, oxaline, and meleagrin, Ōmura et al. demonstrated the use of a nitrone intermediate to access the tethered triazaspirocyclic core of the Penicillium metabolites through iterative intra-molecular cyclizations (Scheme 24) [14, 16, 17].
Scheme 24.
Synthesis of triazaspirocyclic core of roquefortine C-derived metabolites oxaline, mealgrin, and glandicoline B [14].
Sigmatropic rearrangements have been demonstrated to yield triazaspirocycles. A 1,3 sigmatropic rearrangement was shown to take place under thermal conditions to give the triazaspirocycle (Scheme 25) [85]. Oxidative rearrangements have also been reported by Gu et al. and Yamashita et al. [86, 87].
Scheme 25.
Thermal 1,3-sigmatropic rearrangement [85].
6. REACTIONS OF TRIAZASPIROCYCLES
Due to the significant ring strain induced by the triazaspirocyclic core, cycloreversion of triazaspirocycles to the corresponding dipole and dipolarophile is often observed (Scheme 26). Cycloreversion to isocyanates [74, 78, 88, 89], isothiocyanates [90], and azides [66, 91] has been well established, particularly when aromatization results in one or more of the products. Depending on the substitution of the original triazaspirocycle, new triazaspirocyclic systems can be formed from products of cycloreversion, as demonstrated by Richter et al. in 1969 (Scheme 27) [41].
Scheme 26.
Cycloreversion of fused beta-lactams [78].
Scheme 27.
Cycloreversion and cycloaddition of mixed triazaspirocycles [41].
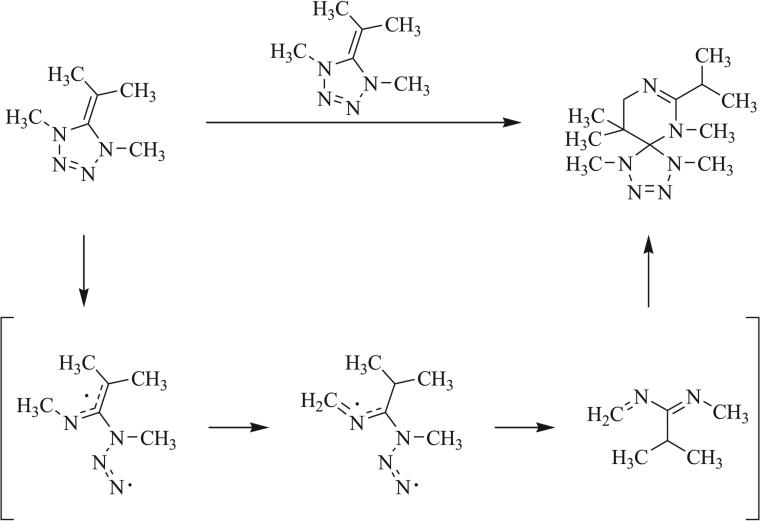
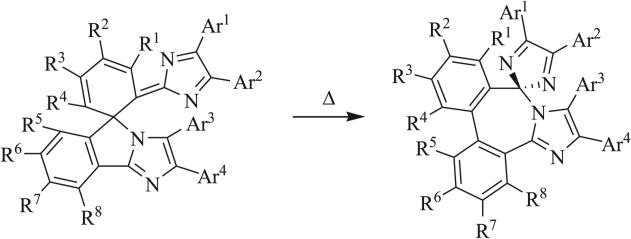
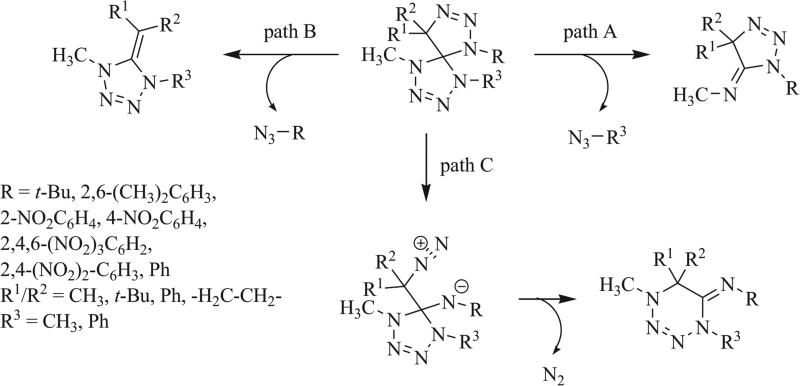
In 1990, Quast et al. reported that thermolysis of a heptaazaspiro[4.4]nonadiene proceeded by one of three mechanisms of cycloreversion (Scheme 28) [66, 91]. [3+2] Cycloreversion was shown to give the triazole (path A) or tetrazole (path B) rings while loss of nitrogen gas (path C) provided the iminotetrahydrotetrazine. The size and electron-withdrawing character of the substituents on the original triazaspirocycle were determined to control the mechanism of cycloreversion. Cycloreversion to the triazole exists in equilibrium with the analogous cycloaddition to the original triazaspirocycle when R1 = R3 = CH3 and R1/R2 were alkyl groups or electron neutral arenes. Irreversible cycloreversion to the tetrazole ring was observed when R = CH3 and R1/R3 were alkyl groups or electron neutral arenes. Competition between path A and path B resulted when R1 = R2 = CH3, R = Ph, and R3 = Ph or CH3. When R was a nitrophenyl species, only path C was observed.
Scheme 28.
Mechanisms of cycloreversion of heptaazaspiro-[4.4]nonadienes [66, 91].
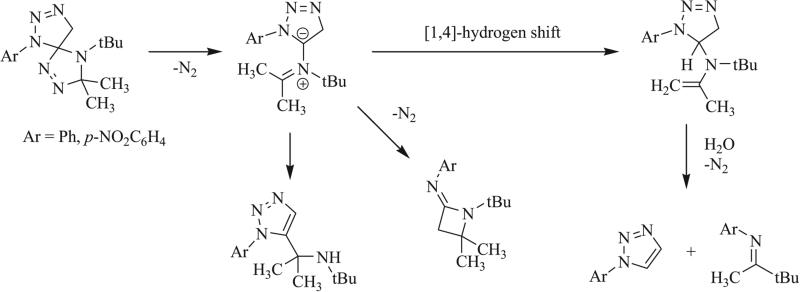
Decomposition of triazaspirocycles has been shown to occur thermally in triazaspirocycles bearing a 1,2,4-triazoline moiety. Schwan et al. observed degradation of this scaffold gave an azomethine ylide upon loss of nitrogen gas (Scheme 29) [67]. This ylide underwent a [1,4]-hydrogen shift to give the 1,2,3-triazoline, which reacted further to undergo heterolytic cleavage and enamine hydrolysis to give the 1,2,3-triazole and amidine. Cleavage of the ylide followed by alkyl shift and loss of nitrogen gas provides the azetidine while rearrangement gives the aromatic triazole product.
Scheme 29.
Thermal decomposition of a triazole-based triazaspirocycle [67].
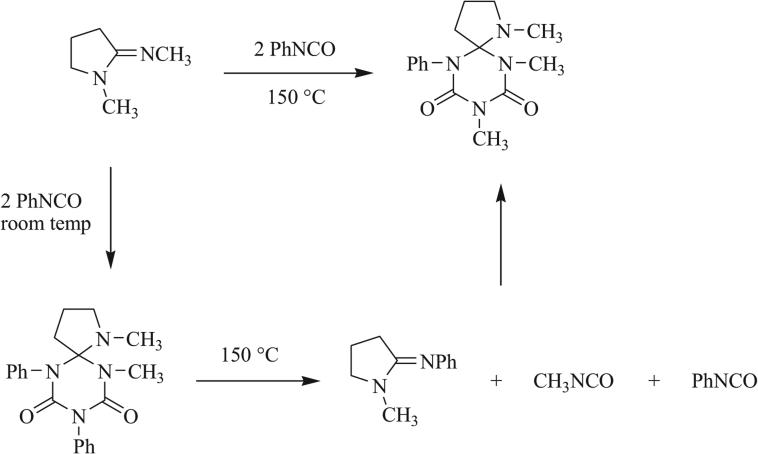
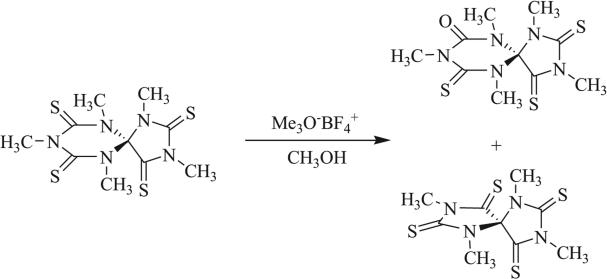
Loss of isocyanate has been demonstrated by Heitz et al., in which attempted methylation of a [4,5] triazaspirocyclic tetrathione led to formation of a urea functionality as well as loss of methyl isocyanate to give the ring contracted [4.4] triazaspirocycle (Scheme 30) [92]. Attempted methylation has also resulted in ring cleavage (Scheme 31) [92].
Scheme 30.
Conversion to urea and ring contraction following attempted methylation [92].
Scheme 31.
Ring cleavage following attempted methylation [92].
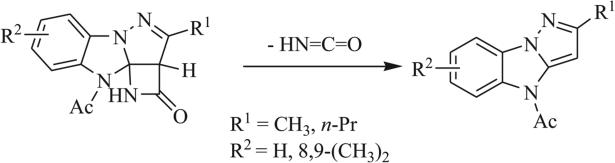
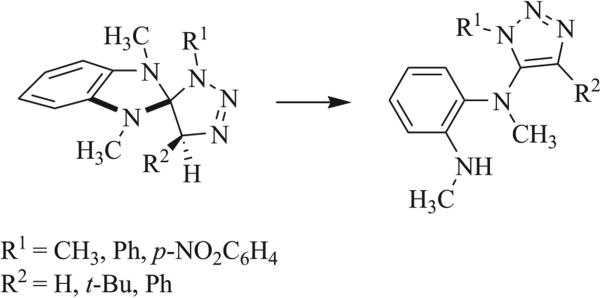
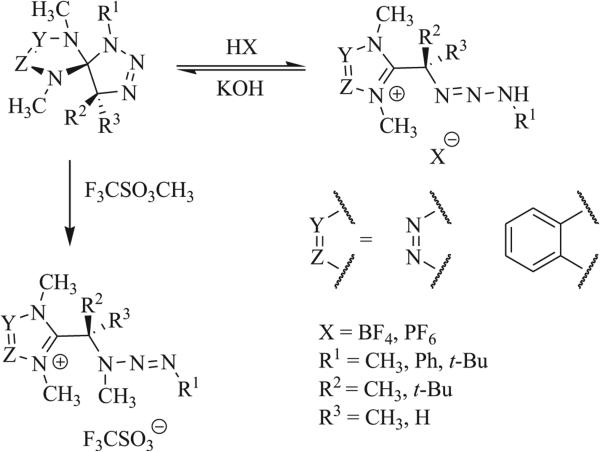
A number of triazaspirocyclic systems are highly susceptible to rearrangement or degradation by hydrolysis, particularly when aromatization results in one or more of the products (Scheme 32) [19, 57, 59, 63, 74, 84, 93]. Methylation-driven ring cleavage has been observed in N,N’-substituted tetrazole- and benzimidazole-based triazaspirocycles (Scheme 33) [93]. Similar ring cleavage was shown to occur in the presence of acid.
Scheme 32.
Rearrangement and aromatization of benzimidazole based triazaspirocycle [63].
Scheme 33.
Acid-promoted and methylation-driven ring opening [93].
7. APPLICATIONS AND OUTLOOK
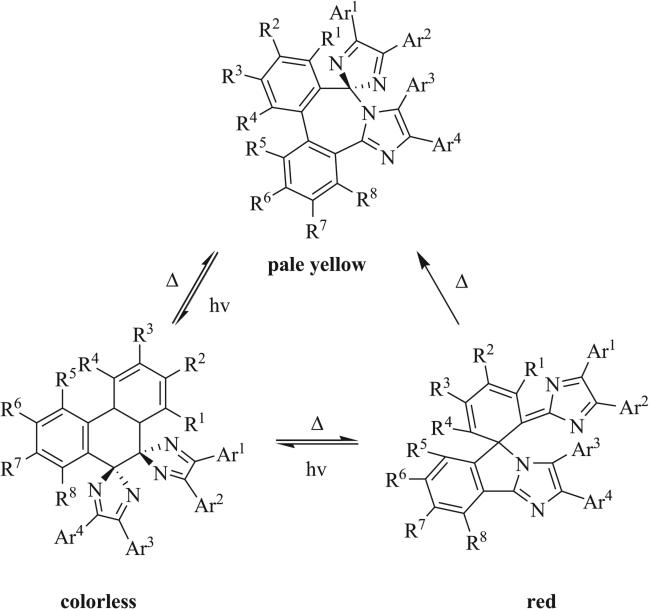
Triazaspirocycles have found use in several applications. In 2013, Horino, Tokita, and Oshima patented a bi-imidazole-based photochromic material which demonstrates negative photo-chromism (Scheme 34) [85]. Photochromic materials undergo reversible color change as a result of isomerization and can be broadly categorized as inducing negative photochromism, a change from colored to uncolored accompanying isomerization, or positive photochromism, a change from uncolored to colored accompanying isomerization. Compounds with photochromic properties have applications in color changing lens and molecular switches, among others. Horino, Tokita, and Oshima found that in compounds where R4 and R5 were bulky groups, irradiation with visible light was shown to cause isomerization from the pale yellow [4.6] triazaspirocycle to the colorless dispirocyclic system. Further isomerization to a red colored [4.5] spirocyclic system was observed when the colorless bispirocycle was shielded from light, after which further isomerization restored the original pale yellow color.
Scheme 34.
Photochromic triazaspirocycles [85].
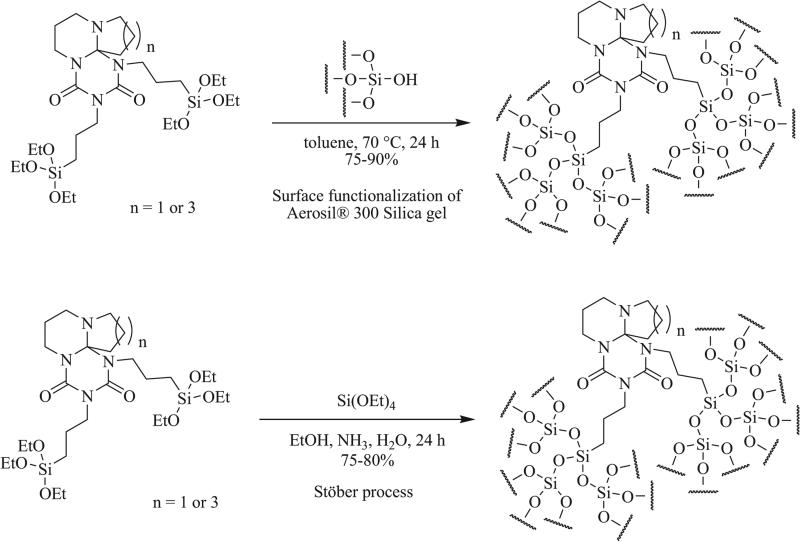
Triazaspirocycles have also demonstrated applications in surface functionalization (Scheme 35) [43]. Following the synthesis of triazaspirocycles via the addition of isocyanates bearing silyl ethers to 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), functionalization of silicon dioxide surfaces was carried out via one of two methods; the first involving the reaction of the silyl ether functionalization triazaspirocycles with fumed silica and the second involving functionalization via the Stöber process [94].
Scheme 35.
Surface functionalization with triazaspirocycles [43].
8. CONCLUSION
In conclusion, triazaspirocycles are unique scaffolds that present promising applications in materials as well as pharmaceuticals. In addition to the previously described material applications, triazaspirocycles have demonstrated antifouling capabilities [10, 35]. Since the International Maritime Organization's ban of highly effective yet environmentally damaging tin antifouling reagents, biofouling has had a negative effect on numerous marine industries through increased fuel consumption, and the need for non-toxic, effective anti-fouling agents is now more urgent than ever. Pharmaceutical applications of triazaspirocycles are promising as well, as the rigid three dimensionality of these complex structures provides excellent potential for improved binding to drug targets. Many natural products have already shown promise in a number of therapeutic areas, such as anti-tumor and anti-microbial drugs. We envision that the continued exploration of the chemistry and bioactivity of these fascinating scaffolds will undoubtedly lead to new advances in several scientific fields.
ACKNOWLEDGEMENTS
The authors acknowledge the financial support of the University of Pennsylvania and the NIH Chemistry-Biology Interface Training Grant.
Biography
 Madeleine M. Joullié
Madeleine M. Joullié
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Sannigrahi M. Stereocontrolled synthesis of spirocycles. Tetrahedron. 1999;55:9007–9071. [Google Scholar]
- 2.Kotha S, Deb A, Lahiri K, Manivannan E. Selected synthetic strategies to spirocyclics. Synthesis-Stuttgart. 2009;2009(02):165–193. [Google Scholar]
- 3.Zheng Y, Tice CM, Singh SB. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014;24(16):3673–3682. doi: 10.1016/j.bmcl.2014.06.081. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann G, Süss-Fink G. Neue [4.5]-Spiroheterocyclen mit CO - NR-Sequenz durch Ruthenium-katalysierte Spirocyclisierung von Isocyanaten. Chem. Ber. 1985;118:3959–3965. [Google Scholar]
- 5.McLeod MC, Brimble MA, Rathwell DCK, Wilson ZE, Yuen T-Y. Synthetic approaches to [5,6]-benzannulated spiroketal natural products. Pure Appl. Chem. 2011;84(6):1379–1390. [Google Scholar]
- 6.Paterson I, Maltas P, Anderson EA. Total synthesis of (+)-spirastrellolide A methyl ester: Challenges and discoveries. Pure Appl. Chem. 2013;85(6):1133–1147. [Google Scholar]
- 7.Aho JE, Pihko PM, Rissa TK. Nonanomeric spiroketals in natural products: structures, sources, and synthetic strategies. Chem. Rev. 2005;105(12):4406–4440. doi: 10.1021/cr050559n. [DOI] [PubMed] [Google Scholar]
- 8.Perron F, Albizati KF. Chemistry of spiroketals. Chem. Rev. 1989;89:1617–1661. [Google Scholar]
- 9.García-Estrada C, Ullán RV, Albillos SM, Fernández-Bodega MA, Durek P, von Döhren H, Martín JF. A single cluster of coregulated genes encodes the biosynthesis of the mycotoxins roquefortine C and meleagrin in Penicillium chrysogenum. Chem. Biol. 2011;18(11):1499–1512. doi: 10.1016/j.chembiol.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Han Z, Sun J, Zhang Y, He F, Xu Y, Matsumura K, He LS, Qiu JW, Qi SH, Qian PY. iTRAQ-based proteomic profiling of the barnacle Balanus amphitrite in response to the antifouling compound meleagrin. J. Proteome Res. 2013;12(5):2090–2100. doi: 10.1021/pr301083e. [DOI] [PubMed] [Google Scholar]
- 11.Zheng CJ, Sohn M-J, Lee S, Kim W-G. Meleagrin, A new FabI inhibitor from Penicillium chryosogenum with at least one additional mode of action. PLoS ONE. 2013;8(11):e78922. doi: 10.1371/journal.pone.0078922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ries MI, Ali H, Lankhorst PP, Hankemeier T, Bovenberg RA, Driessen AJ, Vreeken RJ. Novel key metabolites reveal further branching of the roquefortine/meleagrin biosynthetic pathway. J. Biol. Chem. 2013;288(52):37289–37295. doi: 10.1074/jbc.M113.512665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali H, Ries MI, Nijland JG, Lankhorst PP, Hankemeier T, Bovenberg RA, Vreeken RJ, Driessen AJ. A branched biosynthetic pathway is involved in production of roquefortine and related compounds in Penicillium chrysogenum. PLoS ONE. 2013;8(6):e65328. doi: 10.1371/journal.pone.0065328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada T, Ideguchi-Matsushita T, Hirose T, Shirahata T, Hokari R, Ishiyama A, Iwatsuki M, Sugawara A, Kobayashi Y, Otoguro K, Ōmura S, Sunazuka T. Asymmetric total synthesis of indole alkaloids containing an indoline spiroaminal framework. Chem. Eur. J. 2015;21(33):11855–11864. doi: 10.1002/chem.201501150. [DOI] [PubMed] [Google Scholar]
- 15.Koizumi Y, Arai M, Tomoda H, Ōmura S. Oxaline, a fungal alkaloid, arrests the cell cycle in M phase by inhibition of tubulin polymerization. Biochim. Biophys. Acta. 2004;1693(1):47–55. doi: 10.1016/j.bbamcr.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Sunazuka T, Shirahata T, Tsuchiya S, Hirose T, Mori R, Harigaya Y, Kuwajima I, Ōmura S. A concise stereoselective route to the indoline spiroaminal framework of neoxaline and oxaline. Org. Lett. 2005;7(5):941–943. doi: 10.1021/ol050077y. [DOI] [PubMed] [Google Scholar]
- 17.Ideguchi T, Yamada T, Shirahata T, Hirose T, Sugawara A, Kobayashi Y, Ōmura S, Sunazuka T. Asymmetric total synthesis of neoxaline. J. Am. Chem. Soc. 2013;135(34):12568–12571. doi: 10.1021/ja406657v. [DOI] [PubMed] [Google Scholar]
- 18.Favre HA, Powell WH. Nomenclature of Organic Chemistry. International Union of Pure and Applied Chemistry; Cambridge: 2014. [Google Scholar]
- 19.Antunes AM, Novais DA, da Silva JL, Santos PP, Oliveira MC, Beland FA, Marques MM. Synthesis and oxidation of 2-hydroxynevirapine, a metabolite of the HIV reverse transcriptase inhibitor nevirapine. Org. Biomol. Chem. 2011;9(22):7822–7835. doi: 10.1039/c1ob06052j. [DOI] [PubMed] [Google Scholar]
- 20.Moerdyk JP, Bielawski CW. Alkyne and reversible nitrile activation: N,N'-diamidocarbene-facilitated synthesis of cyclopropenes, cyclopropenones, and azirines. J. Am. Chem. Soc. 2012;134(14):6116–6119. doi: 10.1021/ja3014105. [DOI] [PubMed] [Google Scholar]
- 21.Qu P, Wu ZY, Zhu WM. Redetermined structure of oxaline: Absolute configuration using Cu K-alpha radiation. Acta Crystallogr. Sect. E. Struct. Rep. Online. 2012;68(Pt 6):o1626. doi: 10.1107/S1600536812019423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steyn PS, Vleggaar R. Roquefortine, an intermediate in the biosynthesis of oxaline in cultures of Penicillium oxalicum. J. Chem. Soc. Chem. Commun. 1983;10:560. [Google Scholar]
- 23.Fedoreyev SA, Ilyin SG, Utkina NK, Maximov OB, Reshetnyak MV. The structure of dibromoagelaspongin - a novel bromine-containing guanidine derivative from the marine sponge Agelas sp. Tetrahedron. 1989;45(11):3487–3492. [Google Scholar]
- 24.Reshetilova TA, Vinokurova NG, Khmelenina VN, Kozlovskii AG. The role of roquefortine in the synthesis of alkaloids meleagrine, glandicolines A and B, and oxaline in the fungi Penicillium glandicola and P. atramentosum. Microbiology. 1995;64(1):27–29. [Google Scholar]
- 25.Overy DP, Nielsen KF, Smedsgaard J. Roquefortine/oxaline biosynthesis pathway metabolites in Penicillium ser. Corymbifera: in planta production and implications for competitive fitness. J. Chem. Ecol. 2005;31(10):2373–2390. doi: 10.1007/s10886-005-7107-y. [DOI] [PubMed] [Google Scholar]
- 26.van den Berg MA, Albang R, Albermann K, Badger JH, Daran JM, Driessen AJ, Garcia-Estrada C, Fedorova ND, Harris DM, Heijne WH, Joardar V, Kiel JA, Kovalchuk A, Martín JF, Nierman WC, Nijland JG, Pronk JT, Roubos JA, van der Klei IJ, van Peij NN, Veenhuis M, von Dōhren H, Wagner C, Wortman J, Bovenberg RA. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat. Biotechnol. 2008;26(10):1161–1168. doi: 10.1038/nbt.1498. [DOI] [PubMed] [Google Scholar]
- 27.Hirano A, Iwai Y, Masuma R, Tei K, Ōmura S. Neoxaline, a new alkaloid produced by Aspergillus Japonicus production, isolation and properties. J. Antibiot. (Tokyo) 1979;32(8):781–785. doi: 10.7164/antibiotics.32.781. [DOI] [PubMed] [Google Scholar]
- 28.Overy DP, Phipps RK, Frydenvang K, Larsen TO. epi-Neoxaline, a chemotaxonomic marker for Penicillium tulipae. Biochem. Syst. Ecol. 2006;34(4):345–348. [Google Scholar]
- 29.Kozlovskii AG, Vinokurova NG, Reshetilova TA, Sakharovsky VG, Baskunov BP, Seleznyov SG. New metabolites of Penicillium glandicola var. glandicola: glandicolin A and glandicolin B. Appl. Biochem. Microbiol. 1994;30(3):410–414. [Google Scholar]
- 30.Koolen HHF, Soares ER, Silva F.M.A.d., Almeida R.A.d., Souza ADLD. An antimicrobial alkaloid and other metabolites produced by Penicillium sp. an endophytic fungus isolated from Mauritia flexuosa L. f. Quim. Nova. 2012;35(4):771–774. [Google Scholar]
- 31.Wang J, He W, Qin X, Wei X, Tian X, Liao L, Liao S, Yang B, Tu Z, Chen B, Wang F, Zhou X, Liu Y. Three new indolyl diketopiperazine metabolites from the antarctic soil-derived fungus Penicillium sp. SCSIO 05705. RSC Adv. 2015;5:68736–68742. [Google Scholar]
- 32.Du L, Li D, Zhu T, Cai S, Wang F, Xiao X, Gu Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron. 2009;65(5):1033–1039. [Google Scholar]
- 33.Du L, Feng T, Zhao B, Li D, Cai S, Zhu T, Wang F, Xiao X, Gu Q. Alkaloids from a deep ocean sediment-derived fungus Penicillium sp.; their antitumor activities. J. Antibiot. (Tokyo) 2010;63(4):165–170. doi: 10.1038/ja.2010.11. [DOI] [PubMed] [Google Scholar]
- 34.Nagel DW, Pachler KGR, Steyn PS, Wessels PL, Gafner G, Kruger GJ. X-Ray structure of oxaline: a novel alkaloid from Penicillium oxalicum. J. Chem. Soc. Chem. Commun. 1974;(24):1021. [Google Scholar]
- 35.He F, Liu Z, Yang J, Fu P, Peng J, Zhu W-M, Qi S-H. A novel antifouling alkaloid from halotolerant fungus Penicillium sp. OUCMDZ-776. Tetrahedron Lett. 2012;53(18):2280–2283. [Google Scholar]
- 36.Li XN, Zhang Y, Cai XH, Feng T, Liu YP, Li Y, Ren J, Zhu HJ, Luo XD. Psychotripine: a new trimeric pyrroloindoline derivative from Psychotria pilifera. Org. Lett. 2011;13(21):5896–5899. doi: 10.1021/ol202536b. [DOI] [PubMed] [Google Scholar]
- 37.Ulrich H, Tucker B, Stuber FA, Sayigh AAR. Cycloaddition reactions of isocyanates. Reaction of aryl isocyanates with N,N-dimethylformamide. J. Org. Chem. 1968;33(10):3928–3930. [Google Scholar]
- 38.Dyer E, Majewski TE, Travis JD. Reactions of aryl isocyanates with substituted formamides and formamidines. J. Org. Chem. 1968;33(10):3931–3932. [Google Scholar]
- 39.Ulrich H, Richter R. Mechanism of formation of pentaazadecanetetraones in the reaction of aryl isocyanates with N,N-dimethylformamide. J. Org. Chem. 1971;36(14):2005–2008. [Google Scholar]
- 40.Richter R. Die Addition aromatischer Isocyanate an N-substituierte Amidine. Chem. Ber. 1968;101:3002–3009. [Google Scholar]
- 41.Richter R, Trautwein W-P. Additionen von isocyanaten an nsubstituierte amidine bei erhöhter temperature. Chem. Ber. 1969;102:931–937. [Google Scholar]
- 42.Alsarraf J, Robert F, Cramail H, Landais Y. Latent catalysts based on guanidine templates for polyurethane synthesis. Polym. Chem. 2013;4(4):904. [Google Scholar]
- 43.Schmidt FG, Spange S, Polenz I. Basen/isocyanat-initiierte polymerisation an oxidischen oberflächen. 2013 Aug 15; World Order 117379.
- 44.Giesecke H, Hocker J. Synthese von 1,3,6,9-Tetraaza-spiro[4.4]nonan-2,4-dionen aus 2-Imidazolinen und Isocyanaten. Synth. Stuttgart. 1977;(6):806–808. [Google Scholar]
- 45.Giesecke H, Hocker J, Merten R. Parabansaeureaminale. 1977 Dec; Germany 2625722.
- 46.Giesecke H, Hocker J, Merten R. Parabanic acid aminals. 1979 Aug 7; United States 4163857.
- 47.Matsuoka S.-i., Tochigi Y, Takagi K, Suzuki M. Sequential one-pot and three-component reactions of an N-heterocyclic carbene to form 4-(1,2,4-triazol-5-ylidene)pyrrolidine-2,5-diones: a tandem umpolung/annulation sequence via deoxy-Breslow intermediates. Tetrahedron. 2012;68(47):9836–9841. [Google Scholar]
- 48.Enders D, Breuera K, Runsinka J, Telesb JH. Chemical reactions of the stable carbene 1,3,4-triphenyl-4,5-dihydro-1H-1,2,4-triazol-5-ylidene. Liebigs Ann. Chem. 1996;1996(12):2019–2028. [Google Scholar]
- 49.Schönberg A, Singer E, Stephan W. 1,3-Diphenyl-2-imidazolidinselenon aus 1,1',3,3'-Tetraphenyl Δ2,2' biimidazolidin und Selen. Chem. Ber. 1983;116:2068–2073. [Google Scholar]
- 50.Rigby JH, Wang Z. [4+1] Cycloaddition of N-heterocyclic carbenes with vinyl isocyanates. Org. Lett. 2002;4(24):4289–4291. doi: 10.1021/ol026927g. [DOI] [PubMed] [Google Scholar]
- 51.Cheng Y, Liu MF, Fang DC, Lei XM. Substrate-controlled and site-selective [3+2] cycloadditions of N-heterocyclic carbene derived ambident dipoles. Chem. Eur. J. 2007;13(15):4282–4292. doi: 10.1002/chem.200601482. [DOI] [PubMed] [Google Scholar]
- 52.Katritzky AR, Jishkariani D, Sakhuja R, Hall CD, Steel PJ. Carbene-mediated transformations of 1-(benzylideneamino)benzimidazoles. J. Org. Chem. 2011;76(10):4082–4087. doi: 10.1021/jo200088s. [DOI] [PubMed] [Google Scholar]
- 53.Hoffmann RW, Hagenbruch B, Smith DM. Selektivitat nucleophiler carbene gegenüber heterocumulenen. Chem. Ber. 1977;110:23–36. [Google Scholar]
- 54.Küçükbay H, Durmaz R, Orhan E, Günal S. Synthesis, antibacterial and antifungal activities of electron-rich olefins derived benzimidazole compounds. Il Farmaco. 2003;58(6):431–437. doi: 10.1016/S0014-827X(03)00068-5. [DOI] [PubMed] [Google Scholar]
- 55.Regitz M, Hocker J, Schossler W, Weber B, Liedhegener A. Umsetzungen von bis-[1.3-diaryl-imidazolinylidenen-(2)] mit Iso-, Isothio- bzw. Isoselenocyanaten. Liebigs Ann. Chem. 1971;748:1–19. [Google Scholar]
- 56.Schössler W, Regitz M. Stabile dipole aus 1,1',3,3'-tetraphenyl-2,2'-biimidazolidinyliden und acyliso- bzw. Acylisothiocyanaten. Chem. Ber. 1974;107:1931–1948. [Google Scholar]
- 57.Regitz M, Hocker J. 1,3,6,9-Tetra-spiro[4.4]nonane aus Bi-[1,3-diphenyl-imidazolidinyliden-(2)] und Isocyanaten bzw. Isothiocyanaten. Synth. Stuttgart. 1970:301–302. [Google Scholar]
- 58.Hocker J, Merten R. Parabansaure-Derivate: Ein beitrag zur chemie der nucleophilen carbene. Liebigs Ann. Chem. 1971;751:145–154. [Google Scholar]
- 59.Takamizawa A, Hirai K, Hamashima Y, Sato H. Investigation on the reaction of benzazolium salts with base. Chem. Pharm. Bull. (Tokyo) 1969;17(7):1462–1466. [Google Scholar]
- 60.Étienne A, Lonchambon G, Giraudeau P. 2-Methylene-1,3,5-trimethyl-4,6-dioxo-1,3,5-perhydrotriazine and some methylene-substituted derivatives. C. R. Acad. Sci. II C. 1977;285(9):321–324. [Google Scholar]
- 61.Étienne A, Lonchambon G, Roques J. Some derivatives of 1,3-dimethyl-5-phenyldioxo-1,3,5-perhydrotriazine. C. R. Acad. Sci. II C. 1977;284(20):849–852. [Google Scholar]
- 62.Winberg HE, Coffman DD. Chemistry of peraminoethylenes. J. Am. Chem. Soc. 1965;87(12):2776–2777. [Google Scholar]
- 63.Quast H, Ach M, Hergenröther T, Regnat D. 5-Alkylamino-1H-1,2,3-triazoles by base-mediated cleavage of cycloadducts of azides to cyclic ketene N,N-acetals. Synth. Stuttgart. 2006;2006(12):1943–1945. [Google Scholar]
- 64.Quast H, Bieber L, Meichsner G, Regnat D. [3 + 2]-cycloaddition von methylazid an 5-Alkyliden-1,4-dihydro- 1,4-dimethyl-5H-tetrazole. Chem. Ber. 1988;121:1285–1290. [Google Scholar]
- 65.Quast H, Bieber L, Regnat D. Thermolyse von 5-Alkyliden-1,4-dihydro-5H-tetrazolen. Chem. Ber. 1990;123:1739–1747. [Google Scholar]
- 66.Quast H, Regnat D. Synthese und photolyse von 1,4,4-Trialkyl-4,5-dihydro-5-imino-1H-1,2,3-triazolen Regio und diastereoselektive Bildung von (Z)-Aziridiniminen. Chem. Ber. 1990;123:2195–2202. [Google Scholar]
- 67.Schwan AL, Warkentin J. The synthesis and unimolecular decomposition of four novel Δ1-1,2,4-triazolines. Can. J. Chem. 1988;66:2285–2293. [Google Scholar]
- 68.Tsuge O, Watanabe H, Kiryu Y. The reactions of 1,2-diphenyl-1-azaspiro[2.2]pentane and 2-phenyl-1-azaspiro[2.2]pent-1-ene with C,N- diarylnitrilimines. B. Chem. Soc. Jpn. 1979;52(12):3654–3658. [Google Scholar]
- 69.Buzykin BI, Bystrykh NN. Hydrazones of phthalazones. Some formazans and hydrazidines. J. Org. Chem. USSR. 1983;19(5):953–961. [Google Scholar]
- 70.Yoo KH, Kim DJ, Kim DC, Park SW. Reaction mechanism and structure of new heterocycles via 1,4-dipolar cycloaddition of imidazo[2,1-b]thiazolium-betaine. Heterocycles. 1991;32(2):253–259. [Google Scholar]
- 71.Kim D-C, Kim DJ, Park SW, Yoo KH. Synthesis of new thiazolium betaines and the ring expansioin reaction via 1,4-dipolar cycloaddition. J. Heterocycl. Chem. 1995;32(5):1581–1586. [Google Scholar]
- 72.Trifonov LS, Christova NI, Dimitrov VS, Orahovats AS. Cycloaddition of carbodiimides with a heteroaromatic substituent to allenic acids. Helv. Chim. Acta. 1992;75:1262–1266. [Google Scholar]
- 73.Galal SA, Abdelsamie AS, Soliman SM, Mortier J, Wolber G, Ali MM, Tokuda H, Suzuki N, Lida A, Ramadan RA, El Diwani HI. Design, synthesis and structure-activity relationship of novel quinoxaline derivatives as cancer chemopreventive agent by inhibition of tyrosine kinase receptor. Eur. J. Med. Chem. 2013;69:115–124. doi: 10.1016/j.ejmech.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 74.Fryer RI, Earley JV, Blount JF. Quinazolines and 1,4-benzodiazepines. 77. Reaction of 2-amino-1,4-benzodiazepines with bifunctional acylating agents. J. Org. Chem. 1977;42(13):2212–2219. doi: 10.1021/jo00433a005. [DOI] [PubMed] [Google Scholar]
- 75.Barnes RN, Chambers RD, Silvester MJ, Hewitt CD. Reactions involving fluoride ion. Part 28[1]. Cyclisation and formation of dimers from perfluoro-2,5-diazahexa-2,4-diene. J. Fluorine Chem. 1984;24:211–218. [Google Scholar]
- 76.Coppola GM, Damon RE. The chemistry of 2H-3,1-benzoxazine-2,4(1H)dione (isatoic anhydride). 6. Synthesis of spiro[indoline-2,2'(1'H)quinazolines. J. Heterocycl. Chem. 1979;16:1501–1502. [Google Scholar]
- 77.Ozaki K, Yamada Y, Oine T. Studies on 4(1H)-quinazolinones. 2. Synthesis of 6a,7-dihydro-5H-quinazolino[1,2-a]quinazoline-5,8(6H)-diones. J. Org. Chem. 1981;46(8):1571–1575. [Google Scholar]
- 78.Romano C, Cuesta E.d.l., Avendaño C. Ring contraction of 1,2,4-triazepino[2,3-a]benzimidazol-4-ones. New fused β-lactams. J. Org. Chem. 1991;56(1):74–78. [Google Scholar]
- 79.Kurasawa Y, Kanoh M, Kamigaki Y, Okiyama M, Takada A. Facile synthesis and antifungal activity of 3-substituted 4-amino-8-ethoxycarbonylpyrazolo[5,1-c][1,2,4]triazines and pyrazolo[1',5':3, 4][1,2,4]triazino[5,6-b][1,5]benzodiazepines. J. Heterocycl. Chem. 1988;25:1015–1018. [Google Scholar]
- 80.Mohamed SK, Soliman AM, El Remaily MAA, Abdel-Ghany H. Rapidly and highly yielded synthesis of pyrimidine, dihydropyrimidinone, and triazino[2,1-b]quinazolin-6-ones derivatives. J. Heterocycl. Chem. 2013;50(6):1425–1430. [Google Scholar]
- 81.Feldman KS, Fodor MD. Extending pummerer reaction chemistry. Application to the total synthesis of (±)- dibromoagelaspongin. J. Am. Chem. Soc. 2008;130:14964–14965. doi: 10.1021/ja807020d. [DOI] [PubMed] [Google Scholar]
- 82.Feldman KS, Fodor MD. Extending pummerer reaction chemistry: (±)-dibromoagelaspongin synthesis and related studies. J. Org. Chem. 2009;74:3449–3461. doi: 10.1021/jo900283g. [DOI] [PubMed] [Google Scholar]
- 83.Feldman KS, Fodor MD, Skoumbourdis AP. Synthesis of dibromophakellin, dibromophakellstatin, and dibromoagelaspongin via oxidative cyclization of dihydrooroidin derivatives. Synthesis Stuttgart. 2009;18:3162–3173. [Google Scholar]
- 84.Picon S, Tran HD, Martin MT, Retailleau P, Zaparucha A, Al-Mourabit A. Biomimetically inspired short access to the 2-aminoimidazole-fused tetracyclic core of (±)-dibromoagelaspongin. Org. Lett. 2009;11(12):2523–2526. doi: 10.1021/ol900745c. [DOI] [PubMed] [Google Scholar]
- 85.Horino T, Tokita A, Oshima T. Photochromic material. 2013 Apr 25; United States 0102775.
- 86.Gu X, Zhu X, Wei Y, Wang S, Zhou S, Zhang G, Mu X. CNC-Pincer rare-earth metal amido complexes with a diarylamido linked biscarbene ligand: synthesis, characterization, and catalytic activity. Organometallics. 2014;33(9):2372–2379. [Google Scholar]
- 87.Yamashita H, Abe J. Pentaarylbiimidazole, PABI: an easily synthesized fast photochromic molecule with superior durability. Chem. Commun. 2014;50(62):8468–8471. doi: 10.1039/c4cc03137g. [DOI] [PubMed] [Google Scholar]
- 88.Richter R, Ulrich H. Reaction of phenyl isocyanate with N-methyl-2-pyrrolidinone. J. Org. Chem. 1973;38(15):2614–2617. [Google Scholar]
- 89.Tietz H, Rademacher O, Zahn G. A novel isocyanate reaction -the formation and structure of unexpected cycloadducts. Eur. J. Org. Chem. 2000;2000(11):2105–2112. [Google Scholar]
- 90.Jishkariani D, Hall CD, Oliferenko A, Tomlin BJ, Steel PJ, Katritzky AR. Thermal fragmentation of spirodithiohydantoins: a novel route to NHCs. RSC Adv. 2013;3(6):1669–1672. [Google Scholar]
- 91.Quast H, Balthasar J, Hergenröther T, Regnat D. Thermolyse von 1,2,3,4,6,7,8-Heptaazaspiro[4.4]nona-2,7-dienen, [3+2]-Cycloaddukten von Aziden an 5-Alkyliden-4,5-dihydro-1H-tetrazole. Chem. Ber. 1992;125:2749–2756. [Google Scholar]
- 92.Heitz H, Herrmann G, Süβ-Fink G. Zur Chemie CO- und CS-funktioneller pentaazaspiro [4.5]decane: Ringöffnungs- und Ringverengungsreaktionen. Chem. Ber. 1986;119:2895–2899. [Google Scholar]
- 93.Quast H, Ach M, Regnat D. Triazenes by acid-mediated opening of the dihydro-1,2,3-triazole ring of 1,3-dipolar cycloadducts of organic azides to cyclic ketene N,N-acetals. Eur. J. Org. Chem. 2005;2005(20):4441–4447. [Google Scholar]
- 94.Stöber W, Fink A. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968;26:62–69. [Google Scholar]