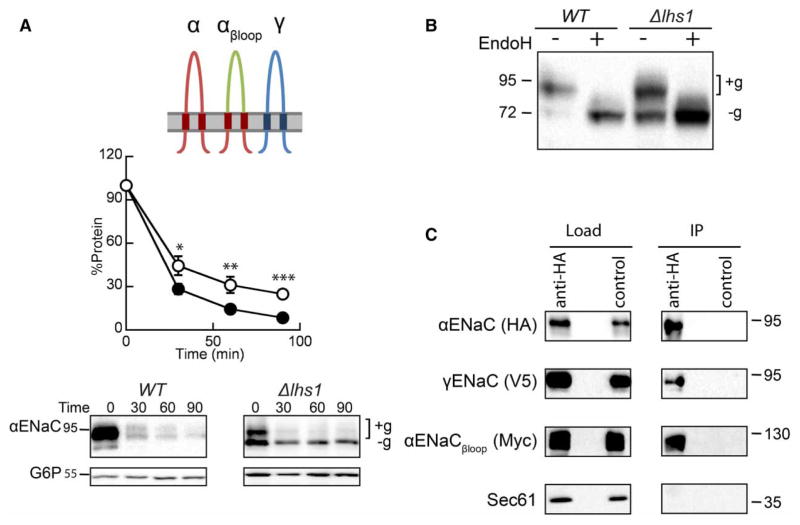
Figure 5. Inserting the α-subunit TMDs and N- and C-termini into βENaC restores Lhs1-dependent degradation of αENaC.
(A) Cycloheximide chase reactions were performed as described in the Experimental Procedures section in WT (filled circles) and Δlhs1 (open circles) yeast strains transformed with plasmids engineered for the expression of αENaC-HA, αENaCβloop-13myc and γENaC-V5. Lysates were prepared, and resolved proteins were immunoblotted with anti-HA antisera (αENaC) and with anti-G6P as a loading control. The expression of αENaCβloop and γENaC were confirmed by western blot (data not shown). Glycosylated (+g) and unglycosylated (−g) species are indicated. Data represent the means of 11–12 experiments, ±SEM. *P < 0.05, **P ≤ 0.01, ***P < 0.001. (B) Cell lysates from WT or Δlhs1 yeast expressing αENaC-HA, αENaCβloop-13myc and γENaC-V5 were treated with Endo H. Anti-HA immunoblots are shown. (C) Cell lysates from WT yeast expressing αENaC-HA, αENaCβloop-13myc and γENaC-V5 were subjected to immunoprecipitation with either anti-HA agarose resin or sepharose (as a control), and resolved proteins were immunoblotted with anti-HA (αENaC), anti-myc (αENaCβloop), anti-V5 (γENaC) or anti-Sec61 as a control. Samples equal to 1% of immunoprecipitated material were also immunoblotted (load).