Abstract
Facial fractures are a common source of emergency department consultations for the plastic surgeon. A working understanding of evaluation, assessment, management, and prevention of further injury when dealing with these fractures is vital. This two-part series detailing the management of midface fractures serves as a guide for the appropriate workup and management of the wide variety of fracture patterns that are commonly encountered.
Keywords: midface fractures, naso-orbital ethmoid fractures, nasal fractures, zygoma fractures, orbital fractures
Epidemiology
The most common causes of facial fractures in the adult population are assaults and motor vehicle accidents (MVAs). Falls, sports, occupational accidents, and gunshot wounds comprise a smaller percentage.1 The underlying insult is largely predictive of a fracture pattern, with MVAs and gunshot wounds contributing to a higher portion of panfacial fractures, sports accidents leading to upper midface fractures, and assault most commonly leading to mandibular fractures.
The advances in automotive safety technology, such as safety belts, have been shown to reduce the incidence of facial fractures and lacerations, whereas non-use of current safety features increases the risk of sustaining facial fractures and panfacial fractures.2 3 In addition, the aging population has led to a higher proportion of facial fractures resulting from falls.2 3 4
Classification of Facial Fractures
In the most basic terms, fractures can be described as either simple or complex, where the former involves only a single anatomical subunit, whereas the latter composes multiple units with well-described patterns. There are many classification schemas when considering facial fractures, but one of the most useful is the Duke classification system.5 This hierarchical system aids in the standardization of fracture terminology and is most useful when there are concomitant, complex facial fractures. As it is hierarchical, lower-order fractures should be described first and any fracture that is included in a recognized fracture pattern should not be listed separately. The hierarchy is listed in Table 1.
Table 1. Duke classification system for the hierarchical ordering of facial fractures.
| Order 1 | LeFort I |
| Order 2 | LeFort II |
| Order 3 | LeFort III Zygomaticomaxillary complex |
| Order 4 | Naso-orbital-ethmoid |
| Order 5 | All simple fractures |
Orbital Fractures
Classification
Four general types of orbital fractures exist: orbital roof fractures, lateral orbital wall fractures, medial orbital wall fractures, and orbital floor fractures. When reporting orbital roof fractures, the affected side, the herniation of orbital contents, and the degree of rim involvement should be described. Lateral orbital wall fractures are rare and should not be listed when part of a zygomaticomaxillary complex (ZMC) fracture or LeFort III hemifracture. Medial orbital wall fractures may have comminution into the rim, lacrimal bone, or orbital floor/roof involvement and as such should be listed. These can occur in isolation, or as a part of a naso-orbito-ethmoid (NOE) fracture. When describing orbital floor fractures, be sure to note the involvement of the infraorbital nerve and the degree of comminution. Also important is the percentage of the orbital floor that is involved in the fracture and herniation of orbital contents into the maxillary sinus. Figs. 1 2 3 illustrate the common radiographic findings of orbital floor fractures.
Fig. 1.
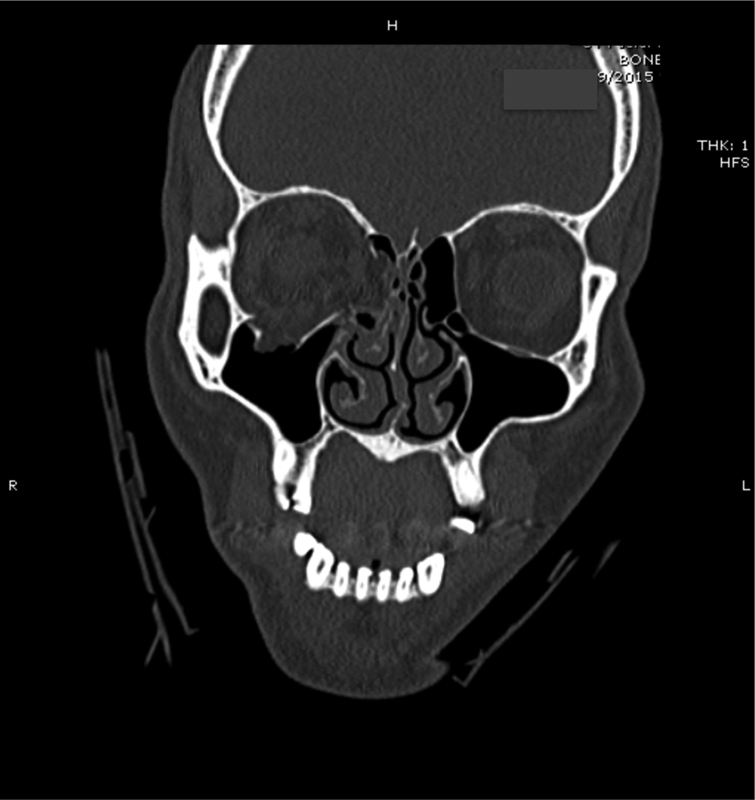
Patient with right-sided orbital floor fracture.
Fig. 2.
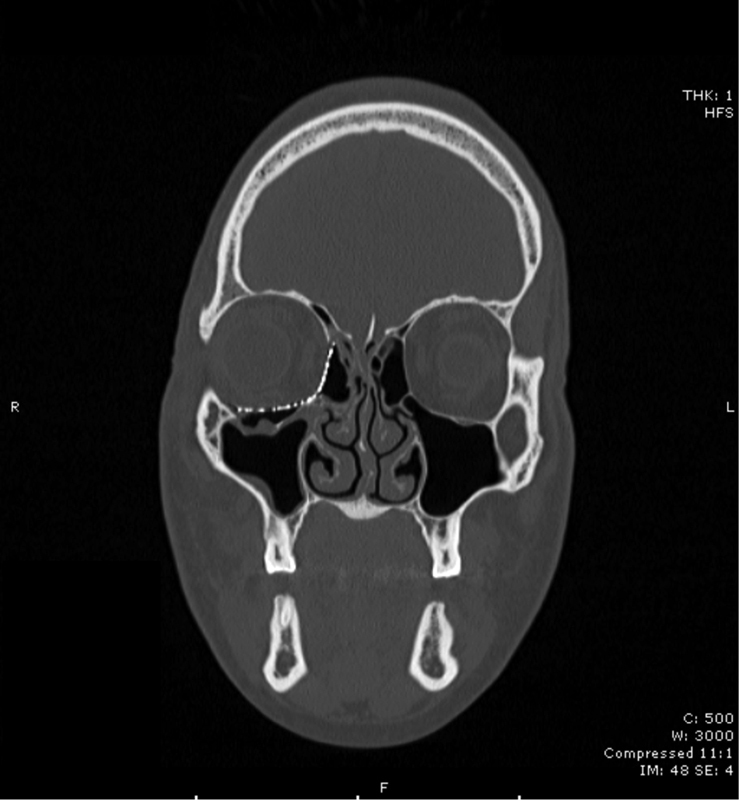
Patient with right-sided orbital floor fracture after reconstruction with an alloplastic implant.
Fig. 3.
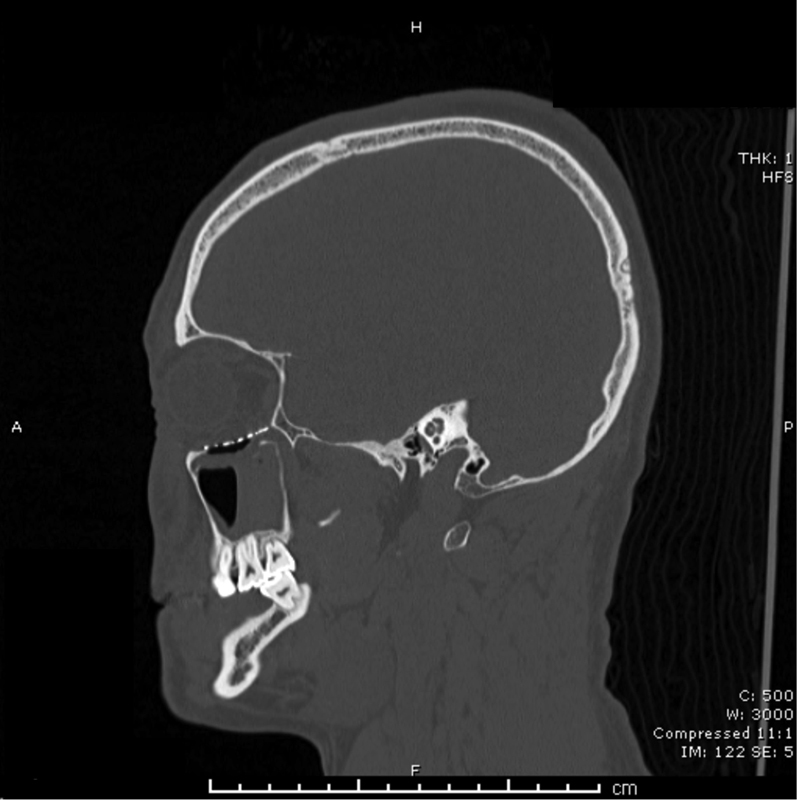
Lateral view of the same patient with right-sided orbital floor fracture after reconstruction.
Physical Examination
Like any other physical examination, the examination of the orbit and surrounding structures should be systematic in nature. Documentation is critical, especially of pretreatment visual acuity. If available before the examination is undertaken, examine radiographs for the guidance of structures that may be involved. Exposure is critical, and as such, dried blood and dirt should be cleaned from the area. Begin the examination with the ocular exam. Check for visual acuity, red-light desaturation, and ocular motility. Snellen eye charts are widely available on mobile phone applications and are a “must-have” for the plastic surgeon. Light and red perception can be tested by shining a flashlight between the first web space and asking the patient if she or he perceives the light as red. Motility can be assessed by asking the patient to follow the examiner's finger and moving the finger in an H-shaped pattern 1 to 2 feet from the patient. If motility is impaired, an orbital fracture should be suspected. A limitation or subsequent diplopia in vertical gaze may suggest either inferior rectus muscle entrapment (upward gaze) or superior rectus muscle entrapment (downward gaze).
Periorbital edema, ecchymosis, and subconjunctival hemorrhage are seen with most orbital fractures. Enophthalmos may suggest a ZMC fracture or any orbital wall fracture. The contour of the orbital rim should be assessed for any step-offs or crepitus. Infraorbital nerve anesthesia prior to the use of a local anesthetic suggests infraorbital nerve compromise and/or an orbital floor fracture. It is vital to check and document for nerve function prior to administering local anesthetic. The medial canthal tendons (MCT) should be assessed; if involved, it may suggest a NOE fracture.
Surgical Indication
Indications for the operative treatment of orbital fractures and lesions are approached as emergent, urgent, or delayed. If a patient requires operative treatment for orbital fractures, it is likely that she will require more urgent operations from other services. However, there are seven conditions that merit emergent operative intervention: the impending loss of vision from trauma to the optic nerve, retrobulbar hematoma, traumatic optic neuropathy, superior orbital fissure syndrome, orbital apex syndrome, trapdoor phenomenon, and symptomatic orbital emphysema. Descriptions of these conditions are provided in Table 2.
Table 2. Emergent surgical indications in orbital trauma and their interventions.
| Condition | Description | Intervention |
|---|---|---|
| Impending loss of vision from trauma to optic nerve | Anatomical disruption of optic nerve fibers from penetrating trauma, bone fragments within the optic canal, or nerve sheath hematomas | Medical or surgical optic nerve decompression |
| Retrobulbar hematoma | Evolving hematomas can encroach upon neurovascular structures and impair vision within an hour. Patients will have pain, proptosis, progressive decline in visual acuity, and CN III palsy. | Decompression of the hematoma via lateral canthotomy and rule out carotid cavernous sinus fistula with imaging |
| Traumatic optic neuropathy | Progressive loss of vision with decreased color perception. There may be optic nerve swelling on CT scan. | Steroid pulse therapy24 and surgical nerve decompression if patient does not have total vision loss on presentation. |
| Superior orbital fissure syndrome | Fracture involvement of the superior orbital fissure with possible injury to CNs III, IV, VI, and V1. Patients may experience ophthalmoplegia, ptosis of the upper lid, proptosis, fixed and dilated pupil, loss of corneal reflex, and loss of conjunctival sensation. | Emergent reduction of fracture |
| Orbital apex syndrome | Fracture involvement of optic foramen leading to ischemic optic neuropathy. Patient may have an APD. | Visual evoked potential testing if APD noted on exam. Emergent reduction of fracture |
| Trapdoor phenomenon | A pediatric condition in which an orbital floor fracture leads to entrapment of the inferior rectus muscle leading to ocular dysmotility, bradycardia, nausea, and syncope resulting from the oculocardiac reflex. | Emergent reduction or orbital contents and reconstruction of the orbital floor |
| Symptomatic orbital emphysema | Central retinal artery occlusion resulting from rising intraorbital air pressure. Patients will experience pain, ocular dysmotility, and visual disturbances. | Needle aspiration for symptomatic rising intraocular pressures. Nose blowing precautions before treatment. |
Abbreviations: APD, afferent pupillary defect; CN, cranial nerve; CT, computed tomography.
Nonemergent cases should be delayed about a week to allow time for swelling to subside. At this point, any diplopia secondary to swelling and orbital tissue contusion should have resolved. Caution should be exercised in waiting longer than 2 weeks as data suggest corrective surgery after this point leads to higher rates of posttraumatic diplopia and a decreased recovery rate.6 For nonemergent cases, general indications include orbital floor defects greater than 1 cm2, or more than 50% of the floor involved (Fig. 1).7 More urgent indications include structural defects, extraocular muscle entrapment, progressive decline in visual acuity, persistent central gaze diplopia, or malposition of the globe. If rounding of the inferior rectus is seen on computed tomography (CT), this suggests entrapment of the muscle. Ultimately, entrapment is a clinical diagnosis on the exam and cannot be confirmed or ruled out on imaging.
Exposure
The approach of the orbit depends on the anatomical subunit needing repair. The superior orbit can be accessed using either the supraorbital or upper eyelid approaches; the inferior orbit and maxilla may be approached using the transconjunctival or transcutaneous lower eyelid approach. See Table 3 for a comparison of approaches.
Table 3. Surgical approaches in orbital trauma.
| Approach (Inferior orbit) | Advantages | Disadvantages |
|---|---|---|
| Transcutaneous (midlid, subciliary) | Superior exposure of inferior rim | Decreased medial wall exposure, ectropion in lax senile lids, visible scarring |
| Preseptal transconjunctival | No visible scarring, easier approach than the retroseptal transconjunctival approach | Need to compromise the orbital septum to get to the orbital floor |
| Retroseptal transconjunctival | Transcaruncular extension allows exposure of medial wall superior to medial canthal tendon, less risk of vertical shortening if septum orbitale is untouched. Retrocaruncular is better than transcaruncular and precaruncular, as there is less postoperative lid complications and extension of exposure to inferior conjunctival fornix.26 Best for blowout fracture access | Dissection can be made difficult by herniation of fat pads into your surgical field |
| Supraorbital | Good exposure of lateral orbital rims, cosmetically favorable result | Disruption of levator aponeurosis or Mueller's muscle can cause ptosis. |
Source: Adapted from Ricketts et al.25
Whichever access is chosen for the orbital floor, the steps are similar once the orbital floor is reached. When reconstructing an orbital floor, elevate the periorbital contents circumferentially from the defect in the floor and identify a stable shelf adjacent to the defect upon which an implant or graft can be placed. When dissecting, be sure to avoid extending in a posterior plane without extending superiorly; otherwise, one runs the risk of violating the maxillary sinus. Once the borders of the fracture are clearly identified, an implant of adequate size must be placed to restore the borders of the orbit (Figs. 2 3). Note that the fracture is not reduced in this case so reconstruction of an orbital floor is a better term to use than reduction.
In accessing the orbital rims, develop an orbital exposure early so that delicate structures can be dissected carefully. When restoring medial and lateral buttresses, the orbital floors and walls are usually the last to be repaired. In addressing the lateral orbital wall, which is formed by the zygomatic and sphenoid bones, zygoma displacement may cause appreciable changes in globe position. Proptosis will result if the zygoma is medially displaced and retrodisplacement of the globe will result if the zygoma is displaced laterally or posteriorly.
Medial orbital walls generally are not accessed unless they are injured in a NOE fracture. The orbital roof is often displaced inferiorly leading to proptosis. Correction of this defect often requires an intracranial approach, and other injuries leading to a persistent cerebrospinal fluid (CSF) leak may necessitate concomitant neurosurgery intervention.
When there is significant comminution, the bony defect may need to be repaired using either a graft or alloplastic materials. Autologous bone is best for large orbital defects adjacent to the ethmoidal and maxillary sinuses as alloplastic implants are more likely to get infected in this area. Alloplastic materials include titanium mesh, porous polyethylene, and a combination titanium mesh with a porous polyethylene coating that allows for easier implantation and malleable shaping.8 Silastic (Dow Chemical Company) should be used with caution as the extrusion rate is high.9
When using alloplastic materials, the implant should be sized appropriately, secured with as few fixation points as necessary, and contoured to fit the defect. The posterior aspect of the implant should be shaped to match the convex nature of the posterior orbital floor to prevent late enophthalmos. After the implant has been fixed in place, a narrow elevator is placed below the implant to ensure the posterior aspect of the implant is superior to the posterior shelf. The two globes are then compared; the corrected globe should have a greater anterior projection compared with the unoperated side to account for surgical swelling. Before closing, a forced duction test is necessary to check for extraocular muscle entrapment.
During the closure, if a lateral canthotomy was performed, the suture canthoplasty is placed on the inner aspect of the orbital rim periosteum at the level of the upper lid with a 4–0 Vicryl (Ethicon, Inc.) suture.7 A Frost suture can be used to support the lower lid.
Postoperative Course, Complications, and Follow-Up
After the procedure, visual acuity is monitored, and corneal protection is maintained. Before being discharged, the patient should be instructed to avoid nose-blowing for 10 days and to avoid airline travel, scuba diving, or any other activity that involves changes in air pressure for 6 weeks to prevent air embolization. Nonsteroidal anti-inflammatory drugs are not recommended for 1 week after the surgery. Currently, there is no evidence suggesting that postoperative steroids are needed. The diligent use of motility exercises is recommended.
Two to 5 days after the operation, the patient should return for removal of the Frost suture, a thorough eye examination, and a check for lower lid retraction. If retraction is encountered, a piece of tape should be applied to the lower lateral lid and retracted to the temple along with vigorous lid massage and forced lid closure exercises. If the retraction is not mitigated with these measures, perform a transconjunctival release of the middle lamella. Further follow-up should take place 4 and 8 weeks after the injury with long-term follow-up to 12 months after the injury.
Nasal Fractures
Classification
Nasal fractures are the most common facial fractures.1 Any fracture that involves the nasal bones, septum, or the nasal process of the maxillary process is considered a nasal fracture. Unlike many of the other facial fractures, there is no uniform classification system, but a nasal fracture can be approached as a bony, cartilaginous, or mixed injury.
Evaluation
The examiner should evaluate for epistaxis, CSF rhinorrhea, swelling, nasal airway obstruction, septal deviation, septal hematoma, and telecanthus. A thorough evaluation of the nose and nasal cavity is often difficult due to extensive swelling and bleeding. Ice may be applied to the swollen areas. Introduce vasoconstrictor sprays into the nares and apply bilateral external pressure to stop bleeding. If these measures have not achieved hemostasis after 15 minutes, tamponade packing will likely be required.
When an isolated nasal fracture is highly suspected, radiography is usually not indicated; however, with the ubiquitous presence of CT scanners, this is unlikely to be the case. A CT scan can be used to guide surgical reduction of the fracture. It is also possible to spot a septal hematoma on a CT scan, but a physical exam remains imperative for this diagnosis.
Surgical Indication
The most common indications for the operative intervention of nasal fractures include septal hematomas, nasal deformity, and nasal obstruction. Airway obstruction is more likely in bilateral fractures and septal displacement.10 Septal hematomas must be treated urgently; otherwise, the hematoma may lead to destruction of the septum and a saddle nose deformity. When discussing the need for surgery with the patient, it is important to consider the mechanism of injury and how likely it is to happen again. For instance, participants in sparring sports are more likely to have recurrent injuries; the goals of reconstruction should therefore be discussed.
If the fracture is to be repaired, it is best repaired within 7 days, with a maximum delay of 14 days. If the fracture is not severe, it can be reduced using a closed technique. Closed reduction of nasal fractures can be accomplished using either local or general anesthesia; however, some suggest that general anesthesia may enhance patient satisfaction of the appearance of the nose. There is no difference in anesthesia modalities when considering the need for retreatment.11
Pledgets soaked with 0.025% oxymetazoline (Afrin, Bayer, Inc.) and 1% tetracaine are introduced into the nasal cavity. After 15 minutes, the area is anesthetized using bilateral infraorbital nerve blocks and bilateral supratrochlear nerve blocks with 1 to 3 mL of 1% lidocaine with 1:100,000 epinephrine. The pledgets are then removed. A Goldman or Boise elevator is placed externally along the nose to measure the distance from the alar rim to the nasion, reducing the likelihood of injury to the cribriform plate. Once done, the elevator should be placed into the nasal cavity against the septum on the side with the concave deformity. The fracture is reduced with the opposite hand, guiding the pieces into alignment. If this is not successful or if it is overcorrected, the process can be completed on the opposite side.
Cartilaginous and septal injuries can be corrected in either an open or closed fashion. First, any septal hematoma must be addressed with a unilateral horizontal mucosal incision at the base of the hematoma and left open to drain. Anterior septal injuries are more likely to require intervention as they may affect the projection of the nose and are more likely than posterior septal injuries to cause obstruction.
Open reduction is best saved for complex nasal bone injuries with severe deviation, significant lacerations, tissue avulsion, acute saddling, and open compound fractures. The injury site can be exposed using either an open septorhinoplasty approach or coronal approach of an overlying laceration. The defect is corrected from proximal to distal. Miniplates and screws are used to anchor free segments at the nasofrontal suture line and the nasal process of the maxilla. If the upper lateral cartilages are avulsed, drill small holes into the nasal bone and use 5–0 clear nylon to secure the cartilage segments.
Postoperative Course, Complications, and Follow-Up
After reduction, external splints and nasal packing are left in place for 5 to 7 days, and cephalexin or amoxicillin should be used for antibiotic prophylaxis. Pain is typically controlled using nonsteroidal anti-inflammatory drugs, ice, and elevation. An oxymetazoline spray could be recommended for the treatment of epistaxis, but the patient must be cautioned to avoid using it for more than 3 days to avoid rhinitis medicamentosa. A nasal spray should be used every 2 hours with thrice daily nasal irrigation with normal saline. The packing is removed within 1 week after the surgery.
Zygomaticomaxillary Complex
Classification
Essential to the understanding of ZMC fractures is that the zygoma has four articulations that can be disrupted. If only one of the frontal, maxillary, sphenoid, or temporal articulations is disrupted, then the fracture is not a true ZMC fracture. Commonly, a radiology report will read “orbital floor fracture, lateral orbital wall fracture, zygomatic arch fracture, and anterior maxilla fracture” when the true classification is simply a ZMC fracture.12 Fractures of the zygomatic arch can be a component of a LeFort pattern, a ZMC fracture, or as an isolated arch fracture. Similarly, fractures at the zygomaticofrontal (ZF) suture can be one part of a more complex LeFort pattern fracture.
Ever since Zingg et al first classified zygomatic fractures, there have been many more attempts to create a classification scheme that would guide discussion and treatment; however, a uniform classifications system has yet to be established.13 14 It is our preference to include the degree of comminution and displacement at each of the fracture sites when describing the fracture. Figs. 4 5 6 demonstrate radiographic findings in isolated zygoma and ZMC fractures.
Fig. 4.
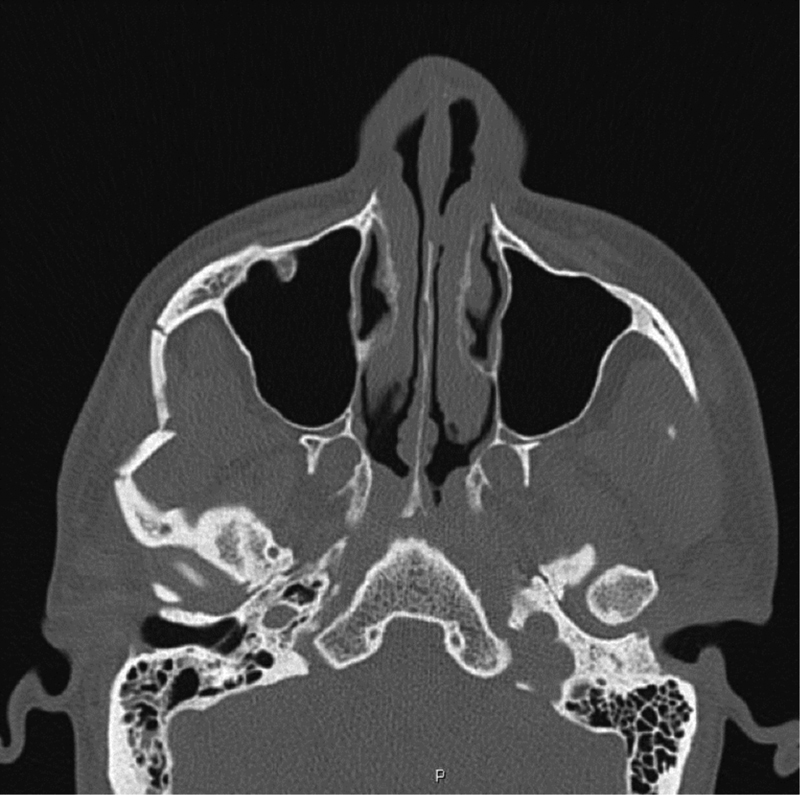
Isolated zygomatic arch fracture. The patient had a closed reduction through a Gilles approach.
Fig. 5.
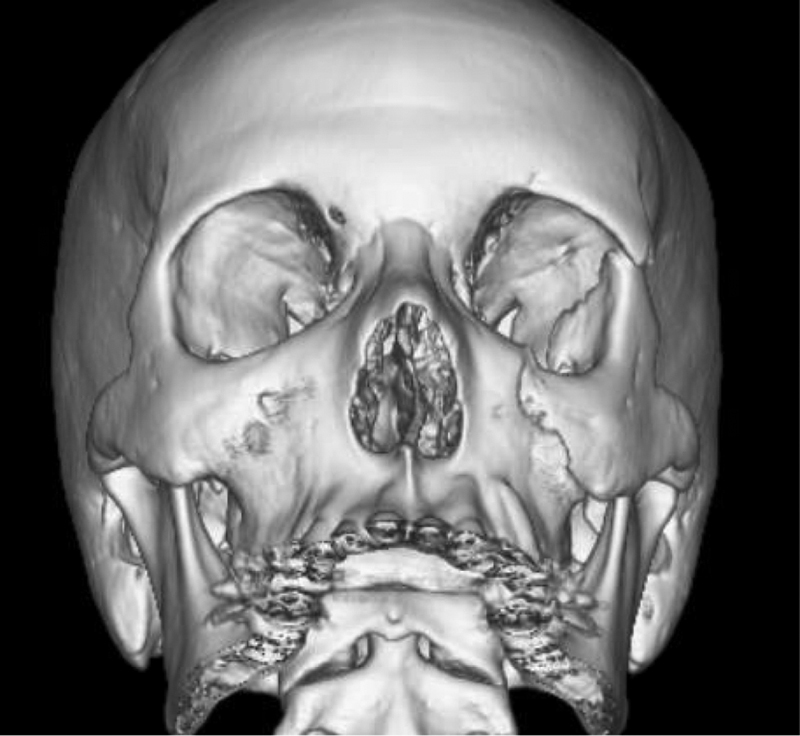
Three-dimensional reconstruction of a 16-year-old male adolescent with a zygomaticomaxillary complex fracture requiring operative fixation for the loss of left malar projection.
Fig. 6.
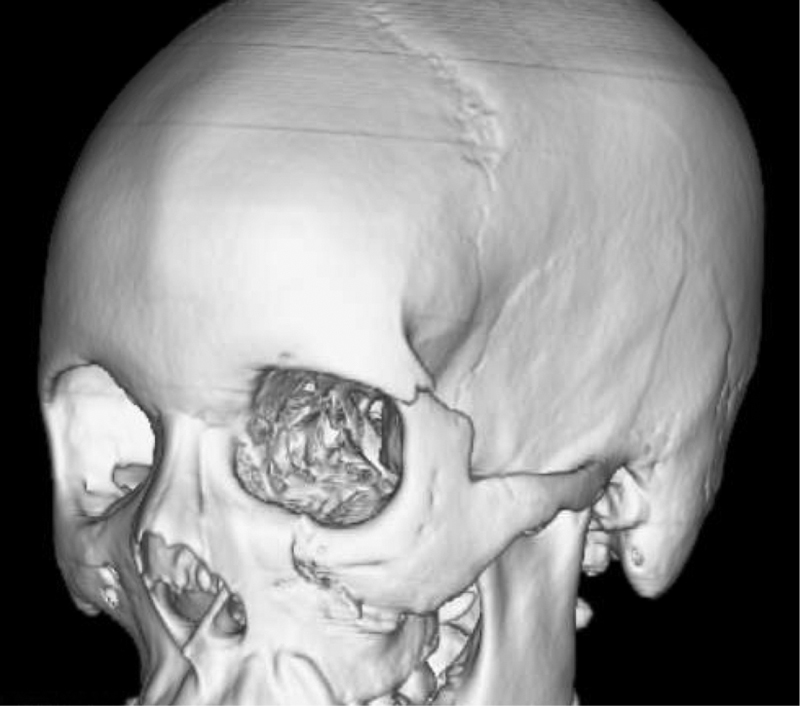
Three-dimensional CTs can assist with operative planning, and provide visual guides when describing an injury to patients during the consultation.
Evaluation
The physical examination should proceed in a systematic fashion starting with a thorough examination of the eye as previously stated. Care again must be taken to evaluate for entrapment, dystopia, and enophthalmos. Bony step-offs can sometimes be palpated at the orbital rims, arch, or maxillary buttress. Swelling can sometimes make this difficult to assess. The lack of bony tenderness at fracture sites suggests that the fracture may not be acute. Numbness in the infraorbital nerve distribution can be seen, as well as neurapraxia of the facial nerve where it passes over the zygomatic arch. A standard maxillofacial CT will confirm the diagnosis and guide operative intervention. Once the edema subsides, there may be a loss of malar projection or a widening of the transverse facial width.
A fracture at the zygomatic arch is rarely a functional problem. In the rare event that the fracture segment interferes with the motion of the coronoid process, this can manifest as trismus and be an indication for surgery. The cosmetic deformity from a zygomatic arch fracture is related to the projection of the malar eminence.15
Operative Intervention
Operative intervention is indicated for cosmetic deformity, enophthalmos, entrapment, dystopia, or other indications for an orbital floor fracture. It should be noted that even though a fracture appears small on the initial CT, it can grow significantly if just one other component is reduced.16
An isolated zygomatic arch fracture can be treated with a Gillies approach, a Keen intraoral approach, or a percutaneous hook.17 The Gillies approach starts two fingerbreadths superior to the zygoma and posterior to the hairline. Dissection is continued down until the plane between the deep portion of the deep temporal fascia and the temporalis muscle is reached. The periosteal elevator is then advanced to the depressed segment of the fracture, and used to reduce the fracture.
When the arch is to be accessed for a full ZMC fracture, it is more commonly accessed via an intraoral approach that also allows access to the zygomaticomaxillary (ZM) and zygomaticosphenoid (ZS) sutures. The approaches to the orbital floor and inferior orbital rim are discussed above. The lateral orbital rim can be accessed through a lateral brow incision, lateral blepharoplasty incision, or an extension of the lower eyelid incision.18
Typically, five points must be evaluated, including the ZF suture, the infraorbital rim, the ZM buttress, the zygomatic arch, and the lateral orbital wall. Once the fractures are exposed, the next step is to achieve reduction. The lateral orbital wall is generally thick and not comminuted; as such, the ZS suture is a reliable marker for obtaining adequate reduction of the whole complex. Of course, if all fractures are open, each should be assessed for good reduction prior to fixation. The Carroll-Girard screw can be used to joystick the whole zygoma in difficult cases; however, care must be taken not to create an avulsion fracture using this tool in those who have bad bone stock (children or the elderly). The orbital floor should be reconstructed last as the size of the defect may grow or shrink with a reduction of the other components.
The amount of fixation that is required depends on the quality of the remaining bone. It is recommended that at least three points of fixation are needed for stability without rotation. The maximum amount of fixation includes a plate at the inferior orbital rim, more inferiorly at the ZM suture, the ZF suture, and the zygomatic arch. The thin skin of the midface only allows for 1.3-mm plates on the orbital rims and a 2.4-mm plate on the zygomatic arch. Larger plates increase the risk of the patient being able to palpate them.19 In children who still have the potential to grow, absorbable plates may be considered to avoid future removal.
Suspension of the midface to the orbital rim at the time of closure is vital to ensure an optimal result. Failing to accomplish this step can lead to ectropion and also signs of aging in the affected half of the face. Frost sutures can also help during the initial period of swelling.
Postoperative Course
Routine postoperative use of antibiotics is not recommended by the senior author. Frost sutures are generally removed at 2 to 5 days. Postoperative imaging is obtained if there are any concerns, but is not ordered routinely. Generally, patients are started on a full liquid diet for 1 week, with advancement to a soft diet for 3 weeks. A regular diet is tolerated after 1 month.
Naso-Orbital-Ethmoid Fractures
Classification
Naso-orbital-ethmoid fractures require a high-velocity direct blow to the upper midface, with the most likely etiologies being MVAs and assault. These forces create five separate fractures: the lateral nose, the inferior orbital rim, the medial orbital wall, the nasomaxillary buttress, and the frontal process of the maxilla.
The most widely accepted classification system of NOE fractures was developed by Markowitz et al and is useful in determining the need for intervention, exposure, and fixation techniques.20 Type I fractures are composed of a single large bone fragment with an intact medial canthus (Fig. 7). The MCT is attached to a comminuted bone fragment in type II fractures. Type III fractures occur when the MCT is avulsed from the lacrimal fossa and bone segment.
Fig. 7.
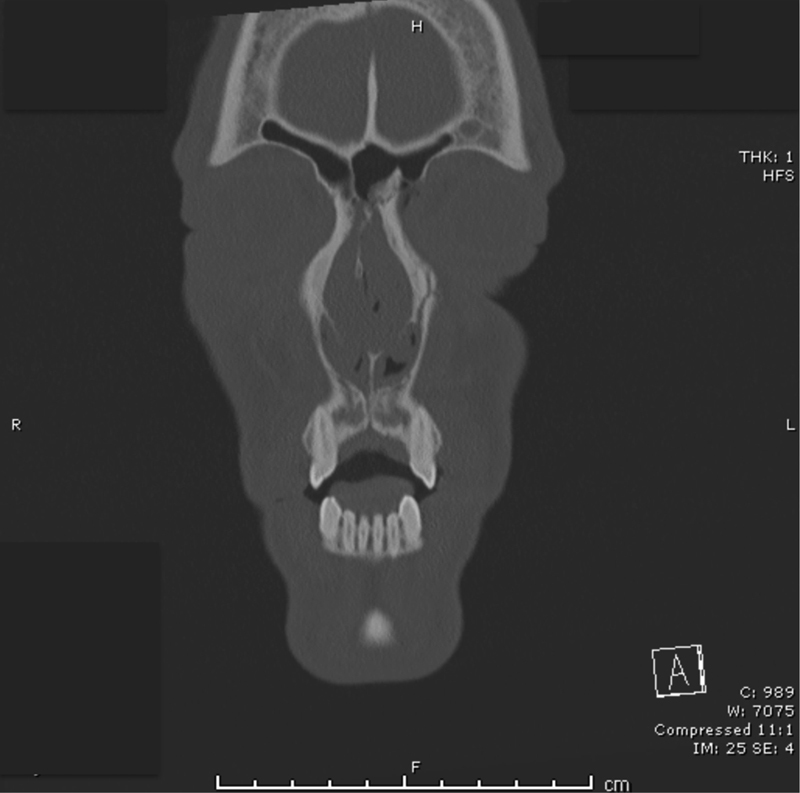
A Markowitz type I left naso-orbital-ethmoid fracture.
Physical Exam and Imaging
Naso-orbital-ethmoid fractures can be appreciated as the posterior displacement of the nasal pyramid or a loss of dorsal nasal support with frank CSF rhinorrhea, telecanthus, periorbital edema and ecchymosis, orbital step-offs, and instability of the canthal tendon. If the examiner is able to elicit crepitus or instability of the canthal tendon with a squeezing pressure between the forefinger and thumb, surgery is indicated. Alternatively, the stability of the MCT can be assessed with intranasal insertion of a Kelly clamp with the tip abutting the medial orbital rim, just above the MCT. Pressure is applied to the Kelly clamp and if instability of the MCT is felt by the contralateral index finger over the area, surgery is indicated. Cerebrospinal fluid rhinorrhea necessitates evaluation by neurosurgery and significant involvement of the orbit necessitates ophthalmology consultation. Considering the substantial degree of force necessary for NOE fractures and the high likelihood of concomitant fractures, a thorough evaluation of the midface and mandible should follow to evaluate for other fractures.3 21
Axial and coronal CT imaging are imperative to evaluate the degree of comminution and the degree of displacement of the medial vertical maxillary buttress. Axial imaging will demonstrate spreading of the medial vertical maxillary buttress.
Surgical Intervention
The surgical correction of NOE fractures is indicated to correct telecanthus, shortened palpebral fissures, nasal airway obstruction, and a soft tissue contour defect of the nasal sidewalls. Four guiding principles should be used when correcting the nasal deformity of a NOE fracture: the rigid fixation of the nasal pyramid and restoration of nasal height and length, the restoration of tip projection, the septal reduction/reconstruction, and the lateral nasal wall augmentation.22 When correcting the defect, the key is overcorrection of the intercanthal distance as undercorrection is difficult to overcome.23
Type I fractures exhibiting no movement on physical exam or showing no evidence of displacement on CT imaging do not require surgical intervention. If indicated, type I fractures can be treated by plate-and-screw fixation of the bony fragments to the adjacent bone. Inferior stabilization can be achieved using a four-hole plate across either the frontal process of the maxillary bone or the inferior rim.23 If there is displacement of the superior aspect of the fracture, the angular process should be stabilized to the nasal process of the frontal bone with a three-hole plate.
Type II fractures involve comminution of the fracture (Fig. 8) and a more challenging dissection; a wider exposure is necessary that can be accomplished using coronal, lower eyelid, and upper gingival buccal incisions. Typically, these fractures require the adjunct usage of miniplate fixation and transnasal wiring. For the wiring, two vertical holes are made anterior to the MCT insertion with 5 mm between each hole. This is repeated on the contralateral side. A 28-gauge wire is passed between these holes and the wire is twisted in the midline until reduction is achieved (Fig. 9). The transnasal reduction is augmented and stabilized using miniplate fixation at the internal angular process of the frontal bone.20
Fig. 8.
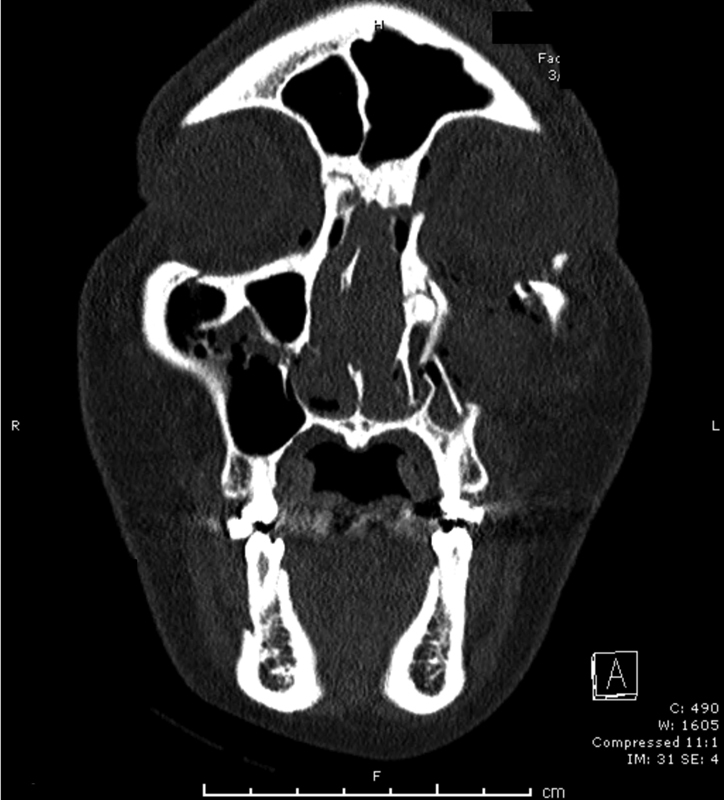
A Markowitz type II naso-orbital-ethmoid fracture.
Fig. 9.
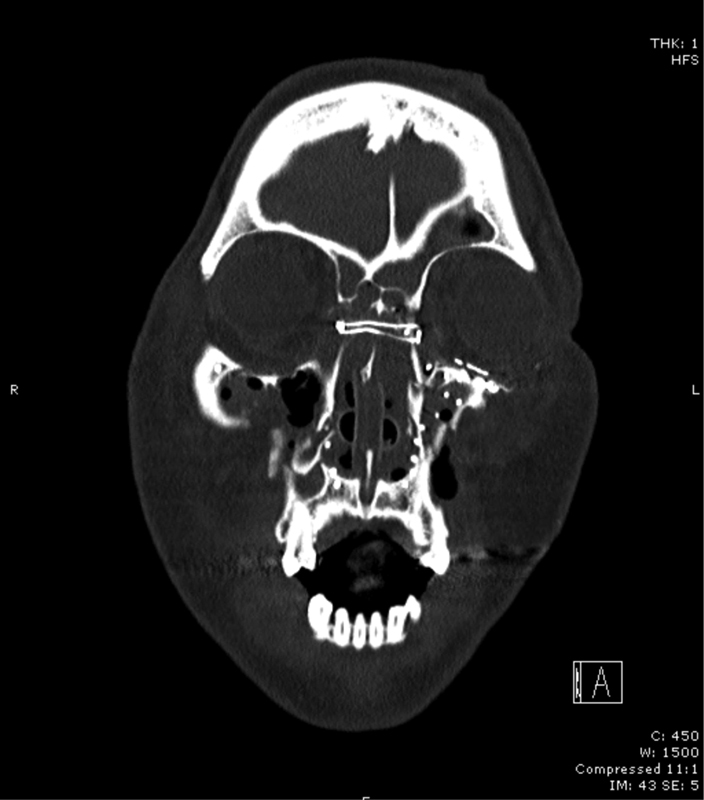
A postoperative view of the transnasal wiring for a naso-orbital-ethmoid fracture.
Correction of type III fractures will typically involve the combination of miniplate fixation and transnasal wiring. Avulsion of the MCT requires reinforcement with wire to the medial orbital wall. Because the central fragment is not likely to have enough surface area for the vertical drill holes for the transnasal wiring, a bone graft should be used from either the medial orbital rim segment or the parietal skull. After properly situating the bone graft, a 4-mm horizontal incision at the eyelid commissure should be made and 3–0 braided suture should be brought through the incision to secure the lateral aspect of the MCT using a modified Kessler stitch. Transnasal wiring should be done as previously described for type II fractures and the suture is connected to the wire. Once again, microplates can be used for reinforcing the reduction.
Postoperatively, an external soft tissue bolster should be placed over the medial canthal valley for 1 week to ensure proper soft tissue contour. If this is neglected, serous fluid accumulation will obliterate the soft tissue contour and yield suboptimal results. Other pitfalls that must be avoided include limited exposure, poor reduction and stabilization, mishandling of the medial canthi, failure to restore nasal contour and projection, and loss of soft tissue contour around the medial canthi.23
Follow-Up
As the mechanism of injury typically results in concomitant injuries, follow-up should be multidisciplinary with other surgical services. Before discharge, the patient should be instructed on proper bolster dressing care and return in 1 week for removal. Subsequently, the patient should return regularly postoperatively for evaluation.
Conclusion
Facial fractures are one of the most common emergency room consultations for the plastic surgeon; as such, a thorough understanding of the regional anatomy, proper physical exam protocols, imaging findings and descriptions, and management is necessary. These guiding principles may serve as a foundation for a more in-depth study of the complex fracture patterns.
References
- 1.Erdmann D, Follmar K E, Debruijn M. et al. A retrospective analysis of facial fracture etiologies. Ann Plast Surg. 2008;60(4):398–403. doi: 10.1097/SAP.0b013e318133a87b. [DOI] [PubMed] [Google Scholar]
- 2.Stacey D H, Doyle J F, Gutowski K A. Safety device use affects the incidence patterns of facial trauma in motor vehicle collisions: an analysis of the National Trauma Database from 2000 to 2004. Plast Reconstr Surg. 2008;121(6):2057–2064. doi: 10.1097/PRS.0b013e31817071fb. [DOI] [PubMed] [Google Scholar]
- 3.Roden K S, Tong W, Surrusco M, Shockley W W, Van Aalst J A, Hultman C S. Changing characteristics of facial fractures treated at a regional, level 1 trauma center, from 2005 to 2010: an assessment of patient demographics, referral patterns, etiology of injury, anatomic location, and clinical outcomes. Ann Plast Surg. 2012;68(5):461–466. doi: 10.1097/SAP.0b013e31823b69dd. [DOI] [PubMed] [Google Scholar]
- 4.Adkinson J M, Murphy R X Jr. Impact of automobile restraint device utilization on facial fractures and fiscal implications for plastic surgeons. Ann Plast Surg. 2011;66(5):472–475. doi: 10.1097/SAP.0b013e318214532c. [DOI] [PubMed] [Google Scholar]
- 5.Follmar K E, Baccarani A, Das R R, Erdmann D, Marcus J R, Mukundan S. A clinically applicable reporting system for the diagnosis of facial fractures. Int J Oral Maxillofac Surg. 2007;36(7):593–600. doi: 10.1016/j.ijom.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Yu D-Y, Chen C-H, Tsay P-K, Leow A-M, Pan C-H, Chen C-T. Surgical timing and fracture type on the outcome of diplopia after orbital fracture repair. Ann Plast Surg. 2016;76 01:S91–S95. doi: 10.1097/SAP.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 7.Sharabi S E, Koshy J C, Thornton J F, Hollier L H Jr. Facial fractures. Plast Reconstr Surg. 2011;127(2):25e–34e. doi: 10.1097/PRS.0b013e318200cb2d. [DOI] [PubMed] [Google Scholar]
- 8.Garibaldi D C, Iliff N T, Grant M P, Merbs S L. Use of porous polyethylene with embedded titanium in orbital reconstruction: a review of 106 patients. Ophthal Plast Reconstr Surg. 2007;23(6):439–444. doi: 10.1097/IOP.0b013e31815a1235. [DOI] [PubMed] [Google Scholar]
- 9.Morrison A D, Sanderson R C, Moos K F. The use of silastic as an orbital implant for reconstruction of orbital wall defects: review of 311 cases treated over 20 years. J Oral Maxillofac Surg. 1995;53(4):412–417. doi: 10.1016/0278-2391(95)90714-9. [DOI] [PubMed] [Google Scholar]
- 10.Chun K-W, Han S-K, Kim S-B, Kim W-K. Influence of nasal bone fracture and its reduction on the airway. Ann Plast Surg. 2009;63(1):63–66. doi: 10.1097/SAP.0b013e31818aebeb. [DOI] [PubMed] [Google Scholar]
- 11.Al-Moraissi E A, Ellis E III. Local versus general anesthesia for the management of nasal bone fractures: a systematic review and meta-analysis. J Oral Maxillofac Surg. 2015;73(4):606–615. doi: 10.1016/j.joms.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Kelley P, Hopper R, Gruss J. Evaluation and treatment of zygomatic fractures. Plast Reconstr Surg. 2007;120(7) 02:5S–15S. doi: 10.1097/01.prs.0000260720.73370.d7. [DOI] [PubMed] [Google Scholar]
- 13.Zingg M, Laedrach K, Chen J. et al. Classification and treatment of zygomatic fractures: a review of 1,025 cases. J Oral Maxillofac Surg. 1992;50(8):778–790. doi: 10.1016/0278-2391(92)90266-3. [DOI] [PubMed] [Google Scholar]
- 14.Ozyazgan I, Günay G K, Eskitaşçioglu T, Ozköse M, Coruh A. A new proposal of classification of zygomatic arch fractures. J Oral Maxillofac Surg. 2007;65(3):462–469. doi: 10.1016/j.joms.2005.12.079. [DOI] [PubMed] [Google Scholar]
- 15.Ellis E III Kittidumkerng W Analysis of treatment for isolated zygomaticomaxillary complex fractures J Oral Maxillofac Surg 1996544386–400., discussion 400–401 [DOI] [PubMed] [Google Scholar]
- 16.Birgfeld C B, Mundinger G S, Gruss J S. Evidence-based medicine: evaluation and treatment of zygoma fractures. Plast Reconstr Surg. 2017;139(1):168e–180e. doi: 10.1097/PRS.0000000000002852. [DOI] [PubMed] [Google Scholar]
- 17.Mavili M E, Canter H I, Tuncbilek G. Treatment of noncomminuted zygomatic fractures with percutaneous screw reduction and fixation. J Craniofac Surg. 2007;18(1):67–73. doi: 10.1097/01.scs.00002467243.31106.81. [DOI] [PubMed] [Google Scholar]
- 18.Zachariades N Mezitis M Anagnostopoulos D Changing trends in the treatment of zygomaticomaxillary complex fractures: a 12-year evaluation of methods used J Oral Maxillofac Surg 199856101152–1156., discussion 1156–1157 [DOI] [PubMed] [Google Scholar]
- 19.Davidson J, Nickerson D, Nickerson B. Zygomatic fractures: comparison of methods of internal fixation. Plast Reconstr Surg. 1990;86(1):25–32. [PubMed] [Google Scholar]
- 20.Markowitz B L, Manson P N, Sargent L. et al. Management of the medial canthal tendon in nasoethmoid orbital fractures: the importance of the central fragment in classification and treatment. Plast Reconstr Surg. 1991;87(5):843–853. doi: 10.1097/00006534-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Becelli R, Renzi G, Mannino G, Cerulli G, Iannetti G. Posttraumatic obstruction of lacrimal pathways: a retrospective analysis of 58 consecutive naso-orbitoethmoid fractures. J Craniofac Surg. 2004;15(1):29–33. doi: 10.1097/00001665-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Potter J K, Muzaffar A R, Ellis E, Rohrich R J, Hackney F L. Aesthetic management of the nasal component of naso-orbital ethmoid fractures. Plast Reconstr Surg. 2006;117(1):10e–18e. doi: 10.1097/01.prs.0000195081.39771.57. [DOI] [PubMed] [Google Scholar]
- 23.Sargent L A. Nasoethmoid orbital fractures: diagnosis and treatment. Plast Reconstr Surg. 2007;120(7) 02:16S–31S. doi: 10.1097/01.prs.0000260731.01178.18. [DOI] [PubMed] [Google Scholar]
- 24.Lai I-L, Liao H-T, Chen C-T. Risk factors analysis for the outcome of indirect traumatic optic neuropathy with steroid pulse therapy. Ann Plast Surg. 2016;76 01:S60–S67. doi: 10.1097/SAP.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 25.Ricketts S, Gill H S, Fialkov J A, Matic D B, Antonyshyn O M. Facial fractures. Plast Reconstr Surg. 2016;137(2):424e–444e. doi: 10.1097/01.prs.0000475760.09451.49. [DOI] [PubMed] [Google Scholar]
- 26.Kempton S J, Cho D C, Thimmappa B, Martin M C. Benefits of the retrocaruncular approach to the medial orbit: a clinical and anatomic study. Ann Plast Surg. 2016;76(3):295–300. doi: 10.1097/SAP.0000000000000531. [DOI] [PubMed] [Google Scholar]


