Abstract
BACKGROUND
Adaptive thermogenesis (AT) is described as a change in resting metabolic rate (RMR) that is greater than would be predicted from changes in lean body mass (LBM) and fat mass (FM) alone during periods of energy imbalance. Whereas an AT-related downregulation of RMR has been implicated in suboptimal weight loss and weight regain following non-surgical weight loss, defense against AT may underpin the durable weight loss following laparoscopic Roux-en-Y gastric bypass (LRYGB) and other bariatric surgeries. However, methodological differences across the few studies that have evaluated post-operative AT limit interpretation as to the effects of these procedures on RMR.
OBJECTIVE
To quantify AT six months after LRYGB and laparoscopic adjustable gastric banding (LAGB).
SETTING
The study was conducted in a large university hospital in the United States.
METHODS
Changes in body composition and RMR were assessed in thirteen severely obese adults six months after LRYGB (n=8) and LAGB (n=5). AT was calculated as the difference between measured RMR and RMR predicted from LBM, FM, age, and sex before and after surgery.
RESULTS
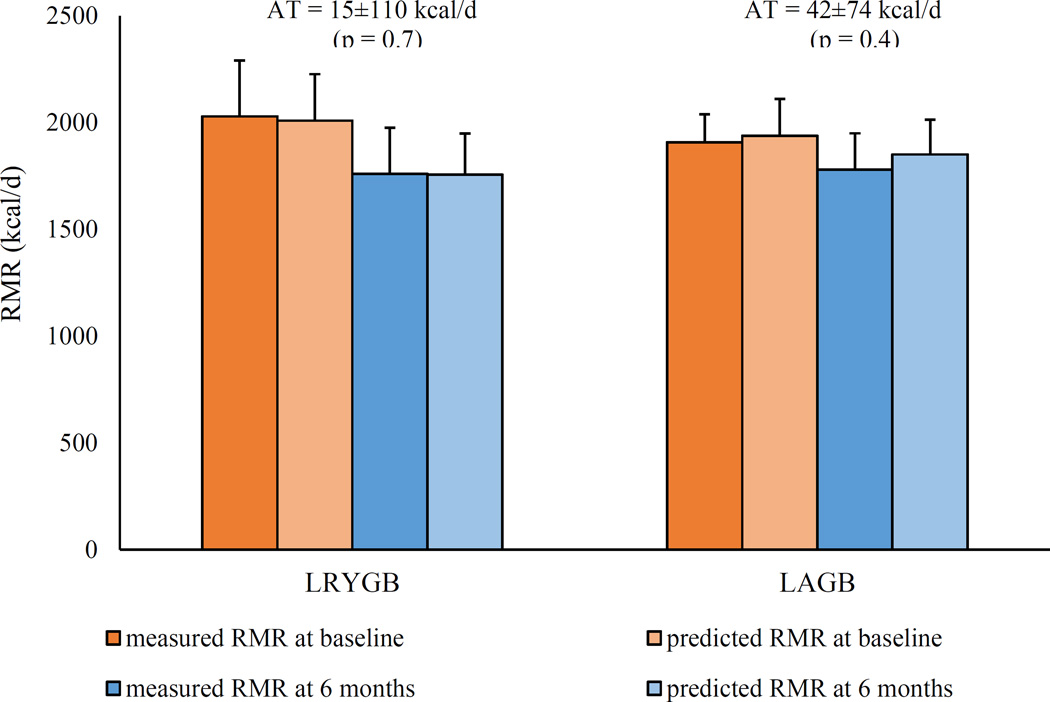
RMR significantly decreased after LRYGB (−270±96 kcal/d, p<0.01) but not after LAGB. Despite significantly greater reductions in body weight, FM, and LBM with LRYGB than LAGB, AT responses after LRYGB (15±110 kcal/d, p=0.7) and LAGB (42±97 kcal/d, p=0.4) were similar (p=0.7).
CONCLUSION
Despite significant body weight and body composition changes, AT was minimal after LRYGB. A blunting of AT may be an additional mechanism that favors sustainable weight loss with LRYGB.
Introduction
Whereas diet and exercise can result in substantial short-term reductions in body weight, consequential declines in energy expenditure challenge the ability of most individuals to lose significant amounts of weight over time (1–3). Resting metabolic rate (RMR) accounts for most of the total daily energy expenditure, indicating the impact of lower metabolic costs of maintaining body tissues on post-weight loss energy balance. In comparison to the goal of optimizing the loss of fat mass (FM), the preservation of more thermogenic lean body mass (LBM) is often encouraged in order to attenuate the weight loss-related decrease in RMR. However, even intense strategies to spare LBM used in the popular weight loss series “The Biggest Loser” were incapable of preventing a significant decline in RMR (4). Rather, reductions in RMR were greater than could be explained by changes in body weight and composition alone, as the regression equations that accurately predicted RMR from LBM and FM prior to the competition significantly overestimated RMR after weight loss (4–6). This energy-sparing phenomenon was initially described as “adaptive thermogenesis” (AT) by Ancel Keys and his colleagues (7) over a half-century ago when they realized that participants in their landmark Minnesota Starvation Experiment demonstrated disproportionately large declines in RMR relative to the depletion of body cell mass after six months of severe caloric restriction. AT is now widely recognized as a dynamic counter-regulatory metabolic response that serves to mitigate changes in body weight during periods of energy imbalance (7–10). Recently, the impact of AT on weight loss outcomes received national attention (front-page headline in The New York Times (11)) as “The Biggest Loser” participants were shown to have significant AT at seven months after initial weight loss and that almost all participants had complete weight regain at the six year of follow-up altogether with unexpectedly and persistent metabolic adaptation (6). In contrast to non-surgical interventions, current bariatric surgery techniques produce substantial and durable weight loss in most patients. Although these procedures result in significant reductions in both FM and LBM and, in turn, RMR, interestingly, some of the limited available evidence suggests that bariatric surgery may defend against energy-sparing with AT (5, 10). For instance, the 240-kcal/d greater decline in RMR observed following seven months of participation in “The Biggest Loser” competition in comparison to six months after laparoscopic Roux-en-Y gastric bypass (LRYGB) was nearly entirely accounted for by a larger AT (5). However, given the greater short-term and long-term weight loss and body composition improvements with LRYGB in comparison to laparoscopic adjustable gastric banding (LAGB) (12), a separate finding that AT was significantly larger six months after LRYGB than LAGB (13) obscures understanding of how AT might impact post-operative energy balance.
Since AT is quantified as the difference between the measured change in RMR and that which would be predicted from changes in metabolically active tissues, differences in the study populations from which the pre-weight loss RMR prediction equations were developed (e.g., both surgical and non-surgical weight loss-seekers (5) versus surgical patients only (13)) and the independent variables included in the RMR prediction equations (e.g., LBM, FM, and age (5) versus LBM, age, and sex (13)) may have influenced the AT calculations in these studies. Therefore, the objective of this study was to quantify AT following LRYGB and LAGB by comparing measured RMR to RMR predicted from LBM, FM, age, and sex before and after surgery.
Methods
Thirteen participants (10 females) from our previously published study population (12) had complete anthropometric and RMR data both before and six months after bariatric surgery and thus constituted the subgroup used in this analysis. Eight participants elected to undergo LRYGB and five LAGB. Eligibility criteria and assessment methods were previously described (12). In short, body composition was assessed before and six months after surgery using dual x-ray absorptiometry (DXA, Hologics Discovery Wi, Bedford, MA, USA), and RMR was measured using open-circuit indirect calorimetry (SensorMedics, Deltatrac II Metabolic Monitor) following an overnight fast. Gas exchange measures were collected for at least 30 minutes with participants resting in the supine position, and the final 20 minutes of stable values were used to calculate RMR. All participants provided written consent prior to study participation. All procedures were approved by the University Committee on Human Research and General Hospital Clinical Research Center Advisory Committee.
Statistical Analysis
We followed the methods of Knuth et al. (5) to predict pre-operative RMR from LBM, FM, age, and sex using least squares linear regression. This same equation was then used to predict RMR six months after surgery. Differences between measured RMR (RMRmeasured) and predicted RMR (RMRpredicted) were compared before and after surgery using independent t-tests. AT was calculated using the established equation (6-month RMRpredicted – baseline RMRpredicted) – (6-month RMRmeasured – baseline RMRmeasured) (8). A more positive value signified greater post- operative energy conservation due to AT, and a one-sample means test was used to determine if AT reached statistical significance in comparison to zero (i.e., no difference between measured RMR and predicted RMR). Normality of all measures of interest was confirmed using Shapiro- Wilks tests. Thus, all values are presented as means and standard deviations. Baseline and six-month differences in anthropometric and RMR measures were examined across all participants and within and between the LRYGB and LAGB groups using paired t-tests.
Results
Patient demographics and body weight, body composition, and RMR before and six months after bariatric surgery are presented in Table 1. Pre-operative body weight, body composition measures, and BMI did not differ between patients in the LRYGB and LAGB groups. LRYGB and LAGB each produced significant reductions in body weight, LBM, and FM, while body fat percentage (BF%) decreased after LRYGB but not LAGB. RMR was significantly reduced after LRYGB only.
Table 1.
Patient descriptives, body weight, body composition, and resting metabolic rate before and six months after LRYGB and LAGB
| All Participants | LRYGB | LAGB | ||||
|---|---|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | Baseline | 6 Months | |
| N (females) | 13 (10) | -- | 8 (6) | -- | 5 (4) | -- |
| Age (y) | 46.2 (12.7) | -- | 46.6 (10.2) | -- | 45.4 (17.3) | -- |
| BMI (kg/m2) | 46.4 (5.8) | 36.8 (6.1) a | 47.1 (6.8) | 34.1 (5.6) a | 45.3 (4.2) | 41.2 (4.3) a b |
| Body weight (kg) | 128.5 (14.6) | 101.9 (15.5) a | 131.7 (15.9) | 95.4 (13.1) a | 123.5 (11.9) | 112.4 (14.1) a b |
| Lean body mass (kg) | 69.9 (12.1) | 61.0 (11.8) a | 71.8 (14.1) | 60.2 (13.3) a | 66.9 (8.6) | 62.4 (10.1) a b |
| Fat mass (kg) | 58.6 (8.9) | 40.9 (11.6) a | 59.9 (9.7) | 35.2 (9.6) a | 56.6 (7.9) | 50.0 (8.3) a b |
| Body fat percentage (%) | 46.6 (5.5) | 40.1 (8.1) a | 46.5 (6.0) | 37.6 (8.9) a | 46.7 (5.1) | 44.1 (5.2) b |
| RMR (kcal/d) | 1982 (222) | 1767 (240) a | 2029 (26.1) | 1759 (287) a | 1907 (13.2) | 1779 (170) |
| RMRpredicted (kcal/d) | 1982 (196) | 1792 (181) a | 2009 (217) | 1755 (193) a | 1938 (172) | 1851 (161) a, b |
| RMRmeasured-predicted | 0 (104) | −25 (114) | 19 (99) | 4 (128) | −31 (116) | −73 (75) |
LRYGB, laparoscopic Roux-en-Y gastric bypass; LAGB, laparoscopic adjustable gastric banding; BMI, body mass index; RMR, resting metabolic rate; RMRpredicted, resting metabolic rate predicted from the equation: RMR (kcal/d) = 1006.775 + 13.614*LBM + 3.888*FM – 5.490*age – 27.569*sex; RMRmeasured-predicted, difference between measured and predicted RMR at each time point
significantly different from before surgery
change from before surgery was significantly different from LRYGB
Adaptive Thermogenesis
In the group as a whole, pre-operative RMR was accurately predicted by the equation: RMR (kcal/d) = 1006.775 + 13.614*LBM + 3.888*FM – 5.490*age – 27.569*sex (r2=0.78, p=0.01) (Table 2). Measured and predicted RMR did not differ significantly before (p=0.59) or after (p=0.10) surgery. AT was not significant after either LRYGB (15±110 kcals/d, p=0.71) or LAGB (42±97 kcals/d, p=0.39) and did not differ between surgeries (p=0.66). Nevertheless, AT was highly variable across individuals, ranging from −143 to 213 kcal/d.
Table 2.
Linear regression model predicting pre-operative RMR
| Model | β | SEE | Standardized β | p value |
|---|---|---|---|---|
| Constant | 1089.483 | 580.612 | 0.097 | |
| LBM | 13.614 | 5.983 | 0.742 | 0.052 |
| FM | 3.888 | 5.232 | 0.155 | 0.479 |
| Age | −5.490 | 4.221 | −0.313 | 0.230 |
| Sex | −27.569 | 164.614 | −0.54 | 0.871 |
RMR, resting metabolic rate; SEE, standard error of the mean; LBM, lean body mass; FM, fat mass
Discussion
The present study demonstrated that substantial reductions in body weight, adiposity and LBM six months after LRYGB did not elicit significant energy-sparing with AT. In support of these findings, Muller et al. (10) found that only 29% of bariatric surgical patients demonstrated AT of at least 120 kcal/d after six months. Similarly, only three of the participants in our study (23%, one who underwent LYRGB and two who underwent LAGB) exhibited this same degree of AT (i.e. > 120 kcal/d). When considering that the loss of FM has been shown to potentiate AT (5, 8, 9), the greater contribution of LBM to weight loss following bariatric surgery in comparison to conventional treatments (14) may at least partially explain the lower AT observed in our participants in comparison to what is often reported following non-surgical weight loss. Alternatively, in an elegantly designed study evaluating AT in non-obese males undergoing severe caloric restriction, Muller et al. (15) found that the loss of LBM but not FM was associated with larger AT. While further studies are needed to understand whether reductions in FM or LBM are the stronger promoter of AT, our finding that AT was minimal after LRYGB and similar in comparison to LAGB, despite significantly greater reductions in LBM, FM, and BF% with the former surgery, suggests that defense against metabolic slowing with AT may be one mechanism that favors sustained weight loss after LRYGB.
The lack of significant AT after LRYGB and LAGB in our participants is in agreement with previous findings that measured and predicted RMR were not different after bariatric surgery (16, 17) but conflicts with some other reports (5, 13). These inconsistencies may be at least partially ascribed to methodological differences across studies. In their comparison of AT in patients who underwent LRYGB to “The Biggest Loser” contestants, Knuth et al. (5) developed the following pre-weight loss RMR prediction equation in a cohort of 52 participants: RMR (kcal/d) = 1026 + 19.8*LBM + 3.1*FM – 2.3*age – 381*intervention group (R2=0.80). In that report, AT was statistically significant in the nine participants who were analyzed six months after LRYGB (201±182 kcal/d). However, the 300-kcal/d greater pre-weight loss RMR in “The Biggest Loser” participants in comparison to the LRYGB patients and the fact that the LRYGB patients who underwent the six-month reassessment accounted for a relatively small proportion (21%) of the study population from which the pre-operative RMR prediction equation was developed might have increased the variability between measured and predicted RMR after surgery and, in turn, inflated AT. We note that in that same study, the measured RMR was slightly greater than the predicted RMR (8±191 kcal/d) in the 13 LRYGB patients who underwent the twelve-month reassessment (5), a value quite similar to the 15±110 kcal/d AT observed in our LRYGB patients after six months. Thus, the latter finding seems to support our supposition that this procedure may protect against AT.
We found that AT did not differ between LRYGB and LAGB. In contrast, Tam et al. (13) reported significant AT in 11 patients after six months with LRYGB (262±62 kcal/d) but not in 8 patients with LAGB (148±85 kcal/d). As with the study by Knuth et al. (5), Tam et al. (13) also did not have complete post-operative data for all participants from which the initial RMR prediction equation was developed. The latter authors (13) assessed body composition utilizing bioelectrical impedance (a less precise method than DXA, as was used in our study) and predicted pre-operative RMR using the equation: RMR (kcal/d) = 1466 + 8.3*LBM – 5.8*age + 113.1*sex (r2=0.32). In comparison, we included FM in our regression model (r2=0.78). Since reductions in adiposity are known to inflate the difference between measured and predicted RMR after weight loss (18), between-surgery differences in FM loss could have influenced their finding of a larger AT after LRYGB than LAGB. Furthermore, Tam et al. (13) calculated AT as the difference between measured and predicted RMR after surgery only, while our AT calculation appropriately took into consideration the differences between measured and predicted RMR both before and after surgery. Thus, it is plausible that the greater precision of our RMR prediction equation and the ability to account for differences in measured and predicted RMR before surgery, as well as the influence of reductions in FM on the post-operative changes in RMR, explain the lower AT values and lack of between-surgery differences in AT in our participants.
These findings help to fill a key knowledge gap regarding how bariatric procedures influence RMR. Most previous investigations have used ratios to assess post-operative changes in RMR relative to body weight or LBM. Despite the widespread use of this approach in the bariatric surgical literature, it is important to recognize that comparisons of RMR per kg of body weight or per kg of LBM before and after weight loss are susceptible to the influence of changes in FM on RMR and, as a result, fail to provide information regarding the impact of AT on RMR following bariatric surgery (19). For example, the loss of FM results in a greater contribution of more metabolically active LBM to the total body weight and, in turn, is directly responsible for the observed increases in RMR per kg of body weight. Such findings may lead to the false conclusion that whole-body thermogenesis is increased following bariatric surgery. In contrast, a lesser contribution of FM to RMR might explain an apparent post-operative reduction in RMR per kg of LBM. Again, this approach cannot differentiate between the influence of FM loss and possible changes in LBM thermogenesis on RMR. For these reasons, we previously cautioned that AT responses following bariatric surgery should be considered separately from evaluations of post-operative changes in RMR per kg of body weight or LBM, as only linear regression- based and not ratio methods can account for the influence of reductions in both LBM and FM on RMR (19). Thus, we interpret our finding of a minimal AT response following LRYGB as a possible contributor to sustained weight loss via a preservation of RMR, independent of the massive weight loss and body composition changes.
Lastly, we emphasize that our and others’ (5, 13) assessments of AT six months after LRYGB and LAGB are in accordance with Keys’ original description of AT following a six-month semistarvation diet (7). While Knuth et al. (5) and Tam et al. (13) further compared measured and predicted RMR at 12 and 24 months, body weight had begun to plateau by these later follow-up assessments. It is important to note that AT is differentially regulated (e.g., by insulin kinetics) from any suppression of RMR that might occur once body weight stabilizes (e.g., by adipocentric signals, such as leptin) (10, 20). Therefore, although longer follow-up studies can provide information regarding AT if body weight is still changing (either gain or loss), studying at steady state may actually provide misleading information regarding the AT response.
Limitations
Although the outcome measures of interest were not different between the LRYGB and LAGB groups at baseline, we acknowledge that the surgeries were not randomly assigned. While our evaluation of AT was restricted to a relatively small number of individuals, our sample size compares favorably to those of previous investigations of AT following bariatric surgery (5, 13). While future controlled studies with larger sample sizes are needed, we believe our findings have internal validity as the ability to accurately predict RMR attenuates the likelihood of sample size affecting our interpretation of the AT response and the precision of our regression model used to predict pre-operative RMR (R2 = 0.78) is comparable to that reported by Knuth et al. (5) (R2=0.80) and far exceeds that of Tam et al. (13) (R2=0.32). Whereas AT may be most pronounced during the dynamic phase of weight loss, we could not exclude the influence of potential between-surgery differences in the rate of weight loss at six months on AT. However, given that AT increases with greater degrees of negative energy balance (5), the greater weight loss across the first six months after LRYGB in comparison to LAGB (13, 21) further suggests a protective effect of LRYGB against AT. We also could not rule out the possibility of differential changes in neurohormonal mediators of metabolism between surgery types. In support of our supposition that LRYGB may protect against AT, others have demonstrated preserved sympathetic support of RMR following LRYGB (22).
Conclusion
The decline in RMR after LRYGB was attributed to the loss of metabolically active tissues, as substantial post-operative reductions in body weight, adiposity, and LBM were not accompanied by significant AT. Alternatively, lesser decreases in body weight and FM may have failed to stimulate significant AT following LAGB. We conclude that a blunting of AT may be an additional mechanism to promote weight loss with LRYGB.
FIGURE 1.
Adaptive Thermogenesis six months after LRGYB and LAGB
Abbreviations: RMR, resting metabolic rate; LRYGB, laparoscopic Roux-en-Y gastric bypass; LAGB, laparoscopic adjustable gastric banding; AT, adaptive thermogenesis.
Values are presented as means and standard deviations (error bars).
Acknowledgments
We thank Dr. Kathleen Mulligan from the School of Medicine at the University of California San Francisco for her thoughtful editorial comments in the drafting of this manuscript.
This research was supported by Grant Number KL2 RR024130 from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research (GMC), and by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
REFERENCES
- 1.Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Heymsfield SB, Harp JB, Reitman ML, Beetsch JW, Schoeller DA, Erondu N, et al. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. The American journal of clinical nutrition. 2007;85(2):346–354. doi: 10.1093/ajcn/85.2.346. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DM, Bouchard C, Church T, Slentz C, Kraus WE, Redman LM, et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes Rev. 2012;13(10):835–847. doi: 10.1111/j.1467-789X.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. The Journal of clinical endocrinology and metabolism. 2012;97(7):2489–2496. doi: 10.1210/jc.2012-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knuth ND, Johannsen DL, Tamboli RA, Marks-Shulman PA, Huizenga R, Chen KY, et al. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity (Silver Spring, Md) 2014 doi: 10.1002/oby.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring, Md) 2016 doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. The Biology of Human Starvation I-II. Minneapolis, MN: University of Minnesota Press; 1950. [Google Scholar]
- 8.Tremblay A, Chaput JP. Adaptive reduction in thermogenesis and resistance to lose fat in obese men. The British journal of nutrition. 2009;102(4):488–492. doi: 10.1017/S0007114508207245. [DOI] [PubMed] [Google Scholar]
- 9.Dulloo AG, Jacquet J. Adaptive reduction in basal metabolic rate in response to food deprivation in humans: a role for feedback signals from fat stores. The American journal of clinical nutrition. 1998;68(3):599–606. doi: 10.1093/ajcn/68.3.599. [DOI] [PubMed] [Google Scholar]
- 10.Muller MJ, Bosy-Westphal A. Adaptive thermogenesis with weight loss in humans. Obesity (Silver Spring, Md) 2013;21(2):218–228. doi: 10.1002/oby.20027. [DOI] [PubMed] [Google Scholar]
- 11.Kolata G. That lost weight? The body finds it. The New York Times. 2016 May 2; [Google Scholar]
- 12.Rabl C, Rao MN, Schwarz JM, Mulligan K, Campos GM. Thermogenic changes after gastric bypass, adjustable gastric banding or diet alone. Surgery. 2014;156(4):806–812. doi: 10.1016/j.surg.2014.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam CS, Rigas G, Heilbronn LK, Matisan T, Probst Y, Talbot M. Energy Adaptations Persist 2 Years After Sleeve Gastrectomy and Gastric Bypass. Obesity surgery. 2016;26(2):459–463. doi: 10.1007/s11695-015-1972-4. [DOI] [PubMed] [Google Scholar]
- 14.Chaston TB, Dixon JB, O'Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. International journal of obesity (2005) 2007;31(5):743–750. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- 15.Muller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, et al. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. The American journal of clinical nutrition. 2015;102(4):807–819. doi: 10.3945/ajcn.115.109173. [DOI] [PubMed] [Google Scholar]
- 16.Coupaye M, Bouillot JL, Coussieu C, Guy-Grand B, Basdevant A, Oppert JM. One-year changes in energy expenditure and serum leptin following adjustable gastric banding in obese women. Obesity surgery. 2005;15(6):827–833. doi: 10.1381/0960892054222768. [DOI] [PubMed] [Google Scholar]
- 17.Carey DG, Pliego GJ, Raymond RL. Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate: six months to one-year follow-up. Obesity surgery. 2006;16(12):1602–1608. doi: 10.1381/096089206779319347. [DOI] [PubMed] [Google Scholar]
- 18.Bosy-Westphal A, Braun W, Schautz B, Muller MJ. Issues in characterizing resting energy expenditure in obesity and after weight loss. Front Physiol. 2013;4:47. doi: 10.3389/fphys.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Browning MG, Franco RL, Cyrus JC, Celi F, Evans RK. Changes in Resting Energy Expenditure in Relation to Body Weight and Composition Following Gastric Restriction: A Systematic Review. Obesity surgery. 2016 doi: 10.1007/s11695-016-2184-2. [DOI] [PubMed] [Google Scholar]
- 20.James Muller M. Adaptive thermogenesis: Do we need new thinking? Obesity (Silver Spring, Md) 2016;24(8):1610–1611. doi: 10.1002/oby.21556. [DOI] [PubMed] [Google Scholar]
- 21.Cottam DR, Atkinson J, Anderson A, Grace B, Fisher B. A case-controlled matched-pair cohort study of laparoscopic Roux-en-Y gastric bypass and Lap-Band patients in a single US center with three- year follow-up. Obesity surgery. 2006;16(5):534–540. doi: 10.1381/096089206776944913. [DOI] [PubMed] [Google Scholar]
- 22.Curry TB, Somaraju M, Hines CN, Groenewald CB, Miles JM, Joyner MJ, et al. Sympathetic support of energy expenditure and sympathetic nervous system activity after gastric bypass surgery. Obesity (Silver Spring, Md) 2013;21(3):480–485. doi: 10.1002/oby.20106. [DOI] [PMC free article] [PubMed] [Google Scholar]