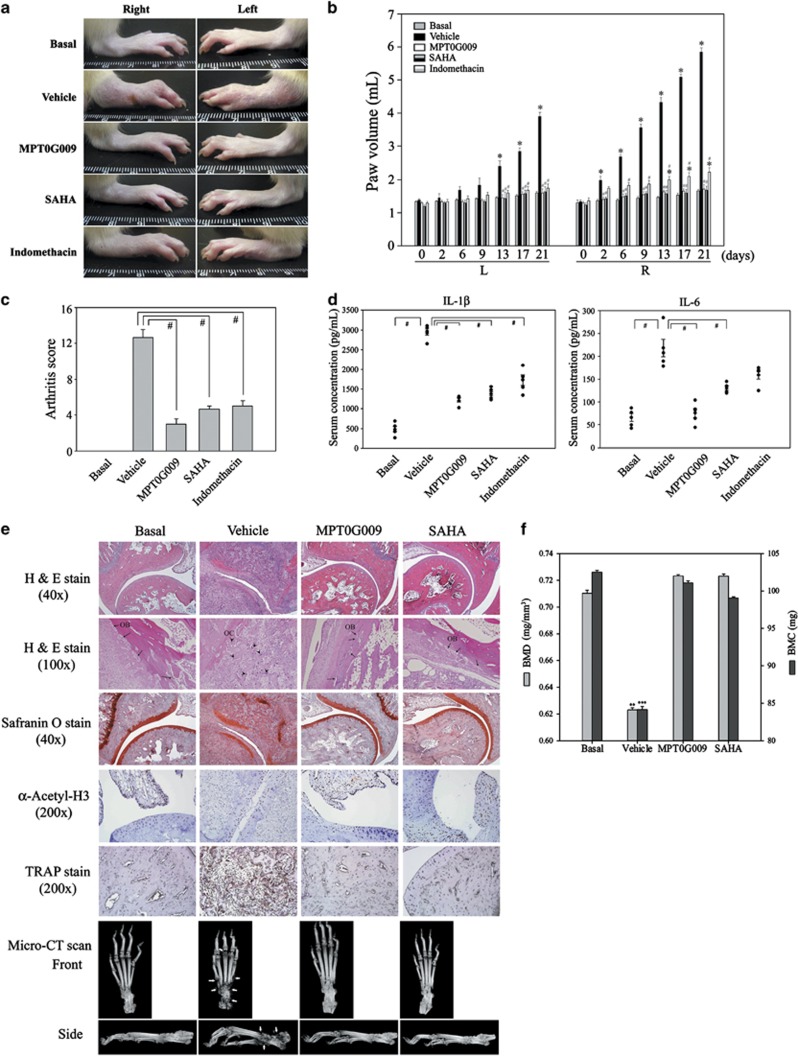
Figure 5.
MPT0G009 inhibits the development of arthritis in an AIA model. (a) After the onset of arthritis (as described in the Materials and methods section), rats were orally treated with either the vehicle (MPT0G009; 25 mg/kg), SAHA (200 mg/kg) or the positive control indomethacin (1 mg/kg) from days 2 to 21 (19 days). Subsequently, swelling of both hind paws was photographed. (b) Hind paw volumes in the indicated group of rats were measured using a digital plethysmometer on the indicated day after AIA induction. (c) Arthritis scores on day 21. (d) Serum levels of interleukin (IL)-1β (left panel) and IL-6 (right panel) on day 21 as measured by enzyme-linked immunosorbent assay (ELISA). (e) Top five rows: photomicrographs of ankle joint sections from the different groups stained with hematoxylin and eosin, safranin O, immunohistochemically stained with an anti-acetyl H3 antibody or TRAP stain. Arrows indicate osteoblasts (OB); arrowheads indicate osteoclasts (OC). Bottom panels: micro-CT results (arrows indicate bone erosion). (f) The BMD (in mg/mm3) and the BMC (in mg) values for the bone tissue of the tarsus were analyzed using CT Analysis Software. Results in (b–d and f) are the means±S.E.M.'s for five independent experiments. *P<0.05 compared with the corresponding day 0 value (b); #P<0.05 compared with the vehicle-treated control (b–d); **P<0.01 and ***P<0.001 compared with basal groups (f)