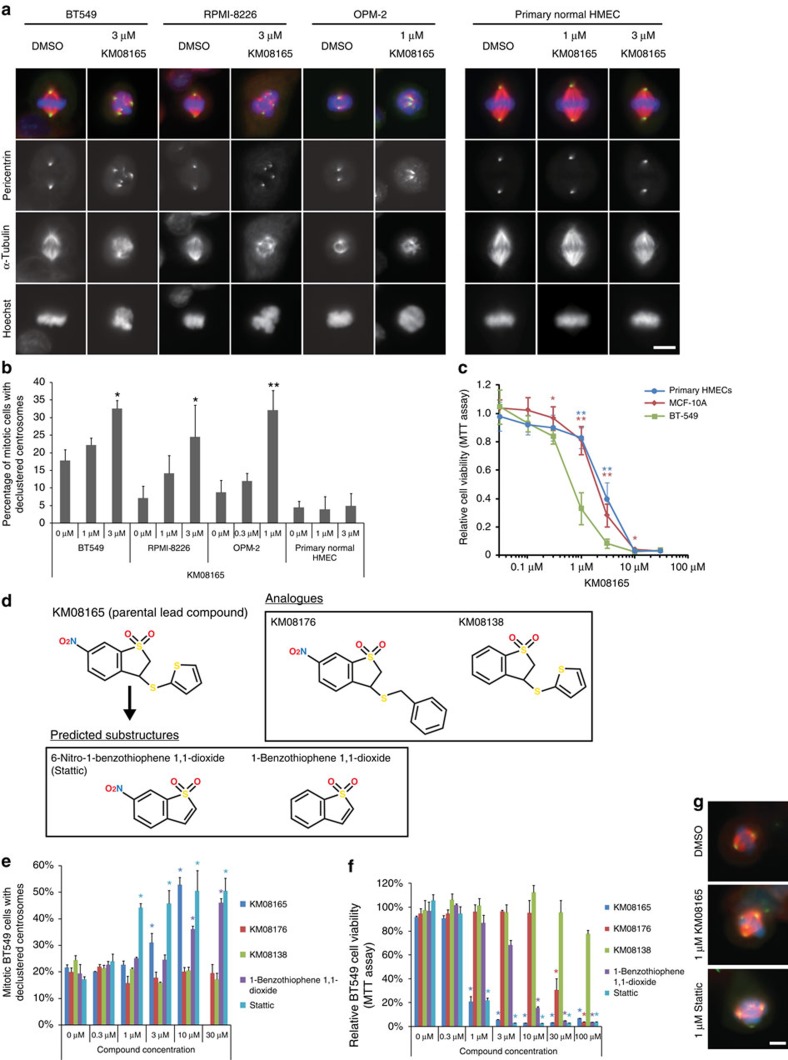
Figure 1. Identification of KM08165 as a centrosome clustering inhibitor and chemical substructure analysis to identify Stattic.
(a) Immunofluorescence images of cells treated with KM08165. Mitotic spindle morphology was observed by staining for pericentrin (green), α-tubulin (red) and DNA (Hoechst, blue). Scale bar, 8 μm. (b) Quantification of KM08165-dependent inhibition of centrosome clustering in cells derived from various origins including breast cancer (BT-549), myeloma (RPMI-8226, OPM-2) and freshly isolated normal primary human mammary epithelial cells (primary HMECs). n=3 biological replicates, ≥128 cells per condition. Statistical significance was tested between untreated and KM08165-treated groups with analysis of variance (ANOVA). (c) Quantification of cell viability 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in invasive mammary tumour cells (BT-549), normal mammary cells (MCF-10A) and primary human mammary epithelial cells (primary HMECs) treated with KM08165. n=3 biological replicates. Statistical significance was tested between BT-549 and the other cell lines at different concentrations of KM08165 with ANOVA. (d) Chemical structures of KM08165 analogues and in silico determined chemical substructures. (e) Percentage of compound-treated BT-549 cells with declustered centrosomes. n=3 biological replicates. Statistical significance was tested between untreated and compound-treated groups with ANOVA. (f) Relative viability of cells treated with KM08165 analogues and computed substructures. n=3 biological replicates. Statistical significance was tested between untreated and compound-treated groups with ANOVA. (g) Images of mitotic cells treated with dimethylsulphoxide (DMSO; control), KM08165 and Stattic. Mitotic spindle morphology was observed by immunofluorescence staining for pericentrin (green), α-tubulin (red) and DNA (Hoechst, blue). Scale bar, 8 μm. *P<0.05; **P<0.01. Error bars represent s.d.