FIG 2 .
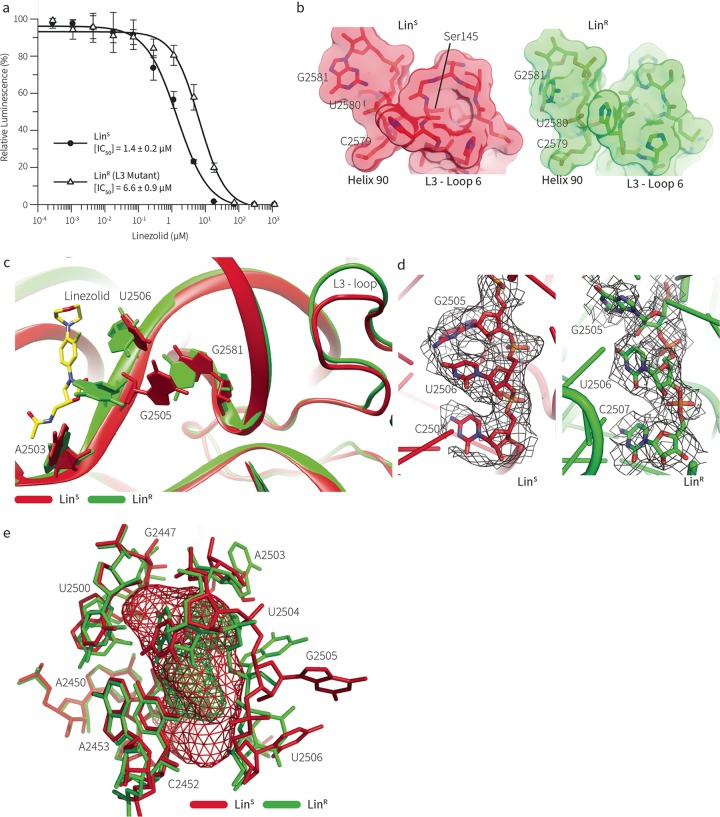
A structural clash that prevents linezolid binding in Linr MRSA. (a) In vitro (IC50) cell-free transcription-translation assay. Data are plotted as the amount of protein synthesis (measured by luciferase translation) versus the concentration of linezolid (in micrograms per milliliter). (b) Representation of the region of uL3 around the site of the ΔSer145 mutation, viewed in the same orientation. The Lins structure is shown on the left (red); the Linr structure is shown on the right (green). The portion of uL3 that interacts with rRNA in “helix 90” is shown. The Linr structure reveals a contraction in the loop of uL3, visible here by the repositioning of His146 (uL3) to become His145 (uL3) in the LinR ribosome, altering the interaction of this loop with helix 90 of the 23S rRNA. (c) The cryo-EM structure of the 70S ribosome from Lins, showing the linezolid position (yellow) and its interaction with 23S rRNA nucleotide G2505 (the position of linezolid is from PDB 4WFA). The cryo-EM structure of the 70S ribosome from Linr is overlaid in green. Deletion of a single amino acid in the uL3 rProtein of the Linr ribosome changes the part of the protein that interacts with an rRNA helix, a helix which in turn makes a direct contact with linezolid. This structural change modifies the position of G2505 in the drug binding pocket, contracting this region and providing fewer contacts to stabilize drug binding. (d) Electron density (drawn at 3σ) around the rRNA nucleotides closest to the linezolid binding site in both the Lins (red, left panel) and Linr (green, right panel), showing the change in orientation of these nucleotides due to the mutation in uL3. (e) Overlay of the cryo-EM structures of the Lins and Linr 70S ribosomes around the binding cavity of the linezolid antibiotic. The cavity available for linezolid binding is shown in the colored mesh. The remodeled Linr binding site (green) is more constricted and less permissive of linezolid binding.