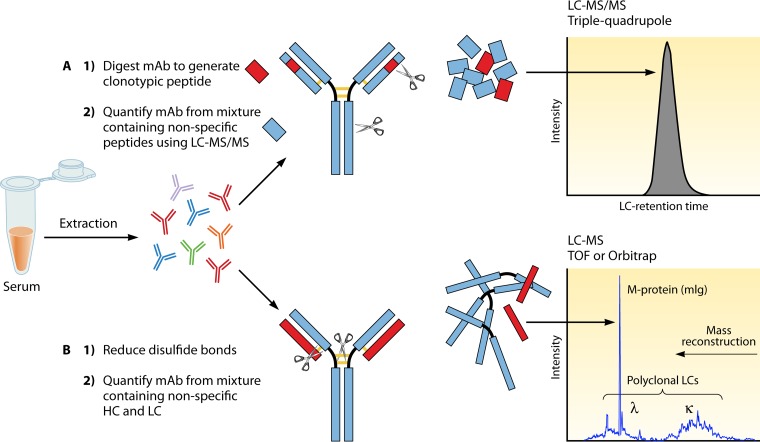
FIG 1.
LC-MS versus LC-MS/MS for quantitation of therapeutic MAbs. MAbs can be quantitated by MS. Ig extraction or enrichment techniques (protein crash, Melon Gel IgG enrichment, or affinity matrix for specific IgG subclasses, for example) will help reduce the protein load in the sample. (A) LC-MS/MS method. The LC-MS/MS method includes separation of the light chains from the heavy chains after reduction of the disulfide bonds, followed by trypsin cleavage to generate multiple peptides from the intact Ig. Peptides specific to the variable region of the MAb on either the light or the heavy chains, which do not cross-react with human sequences, are used to quantitate the MAb. Serum samples are extracted/enriched to reduce the protein load. Samples are denatured (protein is unfolded), reduced (cysteine reduction breaks the disulfide bonds connecting MAb light and heavy chains), alkylated (alkylation of cysteines prevents the disulfide bonds from reforming), and digested by trypsin into smaller peptides. The peptide mixture is separated by LC before analysis by MS/MS. The mass of the peptide of interest (parent ion) is recorded, the peptide is further cleaved inside the mass spectrometer (fragment ion), and the ion pair transition is utilized for quantitation, preferably on triple-quadrupole instruments. (B) LC-MS method. The LC-MS method separates the light chains (LC) from the heavy chains (HC) through reduction, and the intact light chains are not further processed or cleaved. Instead, their accurate mass is measured. In healthy individuals, the polyclonal repertoire of lambda and kappa light chains follows a Gaussian distribution at a 1:2 lambda-to-kappa ratio, and MAbs present in significant amounts stand out of the polyclonal endogenous background as peaks or spikes.