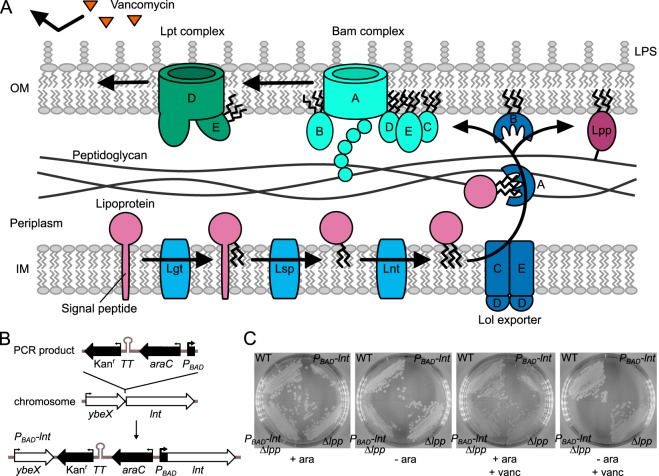
FIG 1.
Lipoprotein maturation in E. coli and complementation strategy. (A) Lipoproteins are sequentially modified by Lgt, Lsp, and Lnt. Once triacylated, lipoproteins are shuttled across the periplasm, where they are inserted into the OM by the Lol export machinery. The Bam complex, catalyzing β-barrel protein assembly and insertion into the membrane, folds LptD in association with LptE, in turn ensuring proper LPS assembly at the outer leaflet of the membrane. The LPS leaflet excludes large hydrophilic molecules, such as vancomycin, from entering the cell. The highly abundant lipoprotein Lpp forms covalent linkages to peptidoglycan through residue K58. (B) The Kanr-TT-araC-PBAD cassette was constructed and inserted directly upstream from lnt by Red-mediated recombination to generate l-arabinose-dependent expression in E. coli. The cassette contained a transcriptional terminator to prevent read-through transcription. (C) Wild-type E. coli strain BW25113 (WT) and PBAD-lnt (strain KA325), Δlpp (strain TXM327), and PBAD-lnt Δlpp (strain KA349) mutants were streaked onto LB agar plates with or without l-arabinose and vancomycin. Plates lacking arabinose contained glucose to suppress basal PBAD expression, while all plates were supplemented with palmitate as a potential source of fatty acids.