Fig. 8.
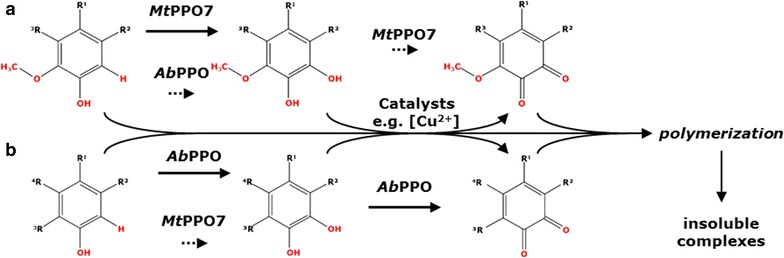
Proposed reaction pathway of MtPPO7- and AbPPO-mediated oxidation of phenolic compounds. a Phenolic compounds comprising a 2-methoxy moiety are hydroxylated by MtPPO7 from M. thermophila C1 at the 6-position. Different from MtPPO7, AbPPO from A. bisporus shows a low efficiency (dotted arrows) towards these methoxylated monophenols. The formed compounds comprising a 1,2-dihydroxy-3-methoxy moiety have, compared to the methoxylated monophenols, a lower electron potential and an increased electron-donating capacity for LPMOs. b Non-methoxylated monophenols are hydroxylated at the ortho-position by AbPPO into compounds comprising a 1,2-benzenediol moiety, whereas MtPPO7 shows a low efficiency (dotted arrows) towards non-methoxylated monophenols. The formed compounds comprising a a 1,2-dihydroxy-3-methoxy and b 1,2-benzenediol moiety are expected to be further oxidized into ortho-quinones by either the MtPPO7- and AbPPO-mediated or catalyst-mediated oxidation, such as copper which was present during the incubation. As indicated in Additional file 6: Figure S5, these ortho-quinones, including intermediate products formed by the oxidation of phenolic compounds, are expected to polymerize and form insoluble complexes
