Abstract
Platelets play an essential role in hemostasis through aggregation and adhesion to vascular injury sites but their unnecessary activation can often lead to thrombotic diseases. Upon exposure to physical or biochemical stimuli, remarkable platelet shape changes precede aggregation or adhesion. Platelets shape changes facilitate the formation and adhesion of platelet aggregates, but are readily reversible in contrast to the irrevocable characteristics of aggregation and adhesion. In this dynamic phenomenon, complex molecular signaling pathways and a host of diverse cytoskeleton proteins are involved. Platelet shape change is easily primed by diverse pro-thrombotic xenobiotics and stimuli, and its inhibition can modulate thrombosis, which can ultimately contribute to the development or prevention of thrombotic diseases. In this review, we discussed the current knowledge on the mechanisms of platelet shape change and also pathological implications and therapeutic opportunities for regulating the related cytoskeleton dynamics.
Keywords: Platelet shape changes, Cytoskeleton dynamics, Thrombosis, Aggregation, Adhesion
INTRODUCTION
Platelets play a key role in hemostasis and thrombosis through aggregation, adhesion and procoagulant activation. However, their unnecessary activation is often linked to life-threatening thrombotic diseases including venous and arterial thrombosis, embolism and stroke (Coller, 2013). Numerous drugs modulating platelet activation have been developed and are hugely successful in the market for the prevention of thrombotic diseases and thrombotic complications in diverse cardiovascular diseases, exemplified by aspirin, Plavix (Bristol-Myers Squibb, NY, USA; clopidogrel), Efient (Lilly, Indianapolis, IN, USA; prasugrel) and Pletal (Otsuka, Tokyo, Japan; cilostazol) (Collins and Hollidge, 2003; Rao et al., 2006). In contrast to aggregation or adhesion, functions that are well-illustrated in the thrombus formation, role of the morphological changes of platelet, i.e., platelet shape change, is relatively less established. Platelets dynamically undergo remarkable morphological alterations when exposed to physical or biochemical stimuli such as high shear or endogenous thrombotic agonists (Hartwig, 2013). Platelet shape changes frequently occur even when the cells are exposed to minimal degrees of perturbation that cannot result in aggregation or adhesion, such as sub-threshold concentrations of platelet agonists or shear stress. In this review, we discuss the current knowledge concerning the mechanisms of platelet shape changes, its toxicological and pathological implications and also the therapeutic opportunities for regulating the associated cytoskeleton dynamics.
PROCESS OF THE SHAPE CHANGES AND CYTOSKELETON DYNAMICS IN PLATELETS
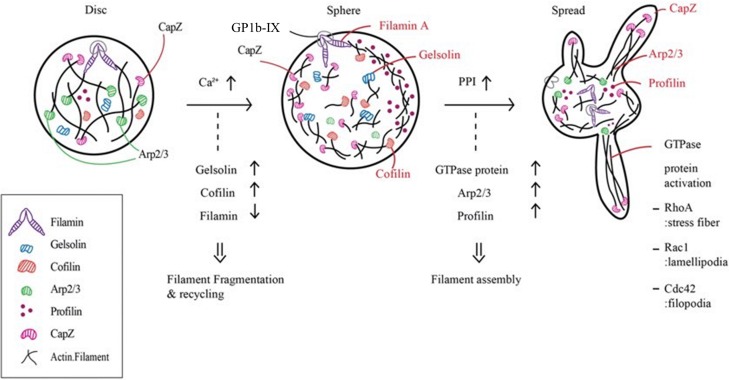
Resting platelets are small discoid shapes around 1.5–3 μm in size. The cytoskeleton of platelets mainly consists of actin, tubulin, spectrin and filamin. Spectrin, which has a two-dimensional web-like structure, laminates the cytoplasmic side of the plasma membrane, both ends of which are connected to actin filaments. Actin exists in polymer where filamin and α-actinin act as cross-linkers with approximately 1:10 ratios (Smyth et al., 2010). In resting platelets, microtubule coils, polymers of αβ-tubulin, are located beneath the plasma membrane, maintaining the discoid shape (Smyth et al., 2010). Upon exposure to various biological stimuli and agonists, shape change occurs via dismantling and reorganization of the cytoskeletons. There are generally two steps in agonist-induced platelet shape change. First, platelets become spherical as actin filaments are fragmented, which is followed by the dismantling and release of spectrin networks composed of filamin A, GP1b/IX and spectrin. Second, the membranes of the cells protrude due to actin filament assembly, which manifests as lamellipodia and filopodia (Sandmann and Koster, 2016). Filopodia protrudes from the core actin fibers while lamellipodia grows from the short actin filament templates (Hartwig, 1992).
Each step in platelet shape change involves the participation of a variety of actin filament-related proteins that are highly concentrated in platelets (Fig. 1). In resting human platelets, the actin filaments from the core to the membrane skeleton are tightly bound to the plasma membrane by GP1b/IX-filamin A complexes (Kovacsovics and Hartwig, 1996). When an agonist binds to its receptor, the activation of phospholipase Cγ induces inositol-1,4,5-triphosphate (IP3) generation and increases intracellular calcium up to 5–10 μM (Berridge et al., 2000; Zheng et al., 2015). Gelsolin is an actin binding protein that severs and caps the barbed-end actin filaments to prevent actin monomer exchange upon intracellular calcium increase in the initial step. Cofilin also binds to actin and contributes to the disassembly of actin filaments and the subsequent release of actin monomers. The actin cross-linking complex, GP1b/IX-filamin, translocates from the plasma membrane to the cytoskeleton during this step. Therefore, severance of actin filaments unlocks the membrane skeleton and induces cell deformation with marginal short actin filaments. This leads to the swelling of the membrane, probably in collaboration with the open canalicular system (OCS), which is considered a reservoir of membrane substances like GP1b/IX and some portion of integrin αIIbβ3 (Smyth et al., 2010) for platelet shape change owing to its invaginated membranous structure connected to the surface. Pplyphosphoinositides (PPI) such as PI-3,4-P2, PI-4,5-P2, and PI-3,4,5-P3 are known to deactivate the capping proteins in actin filaments and expose barbed-end nucleation sites. Also, the Arp2/3 complex stimulates the assembly of actin filaments. There are many actin sequestering proteins such as profilin and thymosin-β4 in platelets that carry an actin monomer to the barbed end and protrude filopodia. Profilin binds to both formin and actin monomers to increase the addition of actin monomers to the barbed end of the filament.
Fig. 1.
Alteration of cytoskeletal proteins during platelet shape change. PPI: polyphosphoinositides.
Meanwhile, intracellular organelles and granules as well as fragmented microtubules are concentrated around the cell center. Finally, the capZ protein recaps the barbed filament ends to complete the assembly and these actin cytoskeletons can be used for cellular contraction, resulting in the final activated platelet shape: spiny spheres with long, thin filopodia extending several micrometers from the platelets.
PLATELET SHAPE CHANGES PRECEDING AGGREGATION
Platelet shape change is regarded as a prerequisite for platelet aggregation. Shape changes of platelets facilitate their adhesion to the site of vascular injury and cohesion with other platelets or erythrocytes, ultimately contributing to the formation of stable aggregates (Jackson, 2007). The degree of platelet aggregation can be easily measured by light transmission aggregometry (LTA) since platelet aggregates consume and precipitate floating platelets, thereby, reducing the turbidity of platelet suspension. In LTA, upon the addition of agonists or exposure to stimuli, light transmission through platelet suspension decreases initially and then increases thereafter. This initial phenomenon observed in LTA, i.e., the decrease of light transmission, is thought to be attributable to platelet shape changes (Maurer-Spurej and Devine, 2001), while some ascribe it to micro-aggregation (Born et al., 1978). It is unclear why platelets change their shapes prior to aggregation, but one explanation is that platelet shape change reduces electrostatic repulsion between two negatively surface-charged platelets.
Many studies have been conducted to understand the mechanisms and interaction of shape change and aggregation. Gαq/PLCβ and PLCγ pathways are important for platelet aggregations activated by thrombin, ADP and collagen, mediated through G-protein coupled receptors, PAR1, PAR4, P2Y1 and GPVI receptor, respectively. The activation of the Gαq/PLCβ pathway results in platelet shape changes through activating IP3-calcium signaling (Offermanns et al., 1997). In Gαq−/− mice, however, while thrombin and collagen induce typical platelet shape changes, aggregation is not triggered, suggesting that the morphological change does not initiate platelet aggregation and the pathways for the two phenomena do not fully coincide. There are some examples of platelet aggregation without preceding shape changes. Again, in the Gαq−/− mice model, platelets did not undergo platelet shape change or aggregation by ADP at the effective dose (10 μM). Interestingly, high concentrations (∼100 μM) of ADP prompted platelet aggregation, presumably due to the Gi2-mediated adenylyl cyclase inhibition (Ohlmann et al., 2000). This suggests that the shape changes are accomplished by the specific interaction between G proteins, Gαq and Gi2, which is distinct from that for the platelet aggregation.
PLATELET SHAPE CHANGES OBSERVED DURING ADHESION
Another distinctive pattern of platelet shape change, spreading to the adhesion molecules, is different from that observed prior to platelet aggregation. While rolling ball-shaped platelets or hemisphere-shaped platelets with filopodia observed before aggregation are reversible, platelets that exhibit a spreading shape, which is flat with a large adhesive surface (platelet diameter >2.5 μm), are terminal. In this case, the cells make more lamellipodia than filopodia, which consists of actin networks cross-linked orthogonally by filamin A (Hartwig et al., 1999). Platelet spreading can further recruit other platelets or neutrophils to increase procoagulant activity. Kuwahara et al. (2002) tried to explain the molecular mechanism occurring during these adhesion steps by testing the effects of the PI3K inhibitor wortmannin, αIIbβ3 blocker c7E3 and an intracellular calcium chelator, dimethyl BAPTA. They reported that while GP1b-vWF (von Willebrand factor) interaction is essential to initiate the platelet shape change with filopodia formation, both αIIbβ3 activation and intracellular calcium increase are necessary for platelet spreading.
SIGNAL TRANSDUCTION FOR PLATELET SHAPE CHANGES
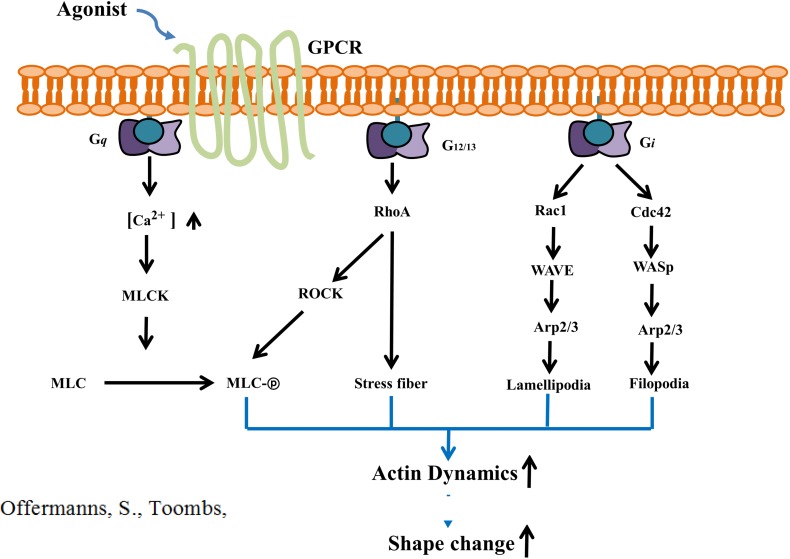
Rho GTPases, Formins, WASp and WAVE
In the platelet, a family of small (∼21 kDa) signaling G proteins, Rho GTPases, RhoA, Cdc42 and Rac1 play key roles in the dynamics of the actin cytoskeleton, platelet aggregation, secretion, and spreading as well as in thrombus formation. It has been reported that Rho GTPase-related actin remodeling, filopodia and lamellipodia formation are mediated through the activation of formins and the Arp2/3 complex (Fig. 2) (Heasman and Ridley, 2008). Cdc42 can induce Arp2/3-mediated filopodia formation through the activation of WASp (Wiskott-Aldrich syndrome proteins) and neuronal N-WASp (Rohatgi et al., 1999). Similarly, Rac1-enhanced lamellipodia formation is related to Arp2/3 activation by the WAVE (WASP-family verprolin-homologous) complex (Miki et al., 1998). Not only WASp and WAVE, but formin proteins (formins) have also emerged as vital effectors of Rho GTPases (Kuhn and Geyer, 2014). Among the 15 formins in mammalian cells, mDia and Daam are shown to be activated upon interaction with Rho GTPases, which causes the central domain for actin polymerization to become exposed and elongated into actin filaments. The Arp2/3 complex provides a platform for new actin polymerization through activating the barbed-end where formin serves as an elongation factor.
Fig. 2.
Signal transduction pathway underlying platelet shape change. WASp: Wiskott-Aldrich syndrome proteins, WAVE: WASP-family verprolin-homologous, MLCK: MLC kinase, ROCK: Rho associated coiled-coil-forming kinase.
Myosin light chain phosphorylation, Calcium, small GTPase proteins
Phosphorylation of the myosin light chain and moesin, an actomyosin filament/plasma membrane cross-linker molecule, is essential for the initiation of shape change in platelets (Daniel et al., 1984; Klages et al., 1999; Retzer and Essler, 2000). Phosphorylation of adhesion site proteins such as myosin facilitates their binding to actin filaments, ultimately generating contractile force on the actin framework. Previously, it has been reported that both calcium dependent and independent mechanisms result in myosin light chain phosphorylation (Paul et al., 1999). Agonists induce intracellular calcium influx through the Gαq/PLCγ-IP3 pathway and increased calcium can cooperate with calmodulin to activate MLC kinase (MLCK) (Fig. 2). PLCγ activation is mediated by the binding of Syk kinase to the ITAM (immunoreceptor tyrosine-based activation motif) of the GPVI and FcγRIIa receptors and the association of adaptor proteins, LAT and SLP-76. Shape changes also occur in the absence of calcium peaks. Calcium-chelated cells were observed to elongate and form abnormal filopodia (Hartwig et al., 1995). Even without calcium peaks, calcium-independent RhoA can activate Rho associated coiled-coil-forming kinase (ROCK), which induces shape changes directly by phosphorylating MLC or indirectly through inhibiting myosin phosphatase (PP1M).
ACTIVATORS OF PLATELET SHAPE CHANGES
Physiological stimuli
Prior to aggregation, many agonists such as ADP, platelet- activating factor (PAF), 5-hydroxytryptamine (5-HT, serotonin), thrombin and collagen induce remarkable morphological changes (Maurer-Spurej and Devine, 2001). Previously, Sanderson et al. (1996) determined the time and dose dependency forward/side-scatter profile of platelets following exposure to platelet activating agonists using a flow cytometer. Depending on the agonist exposed, the onset of platelet forward/side scatter changes was different. For instance, ADP, PAF and 5-HT initiated platelet shape changes rapidly and strongly, whereas other agonists like A23187 and collagen induced strong but slower responses. Interestingly, although each agonist could induce both platelet shape change and aggregation, it is not always mediated via a single receptor activation. For instance, a selective P2Y purinoceptor antagonist ARL66096 was effective against aggregation but had no effect on ADP-induced platelet shape changes, indicating that two different receptors and distinct signaling pathways could be involved in agonist-stimulated platelet activation. Epinephrine is well known for its potentiating effects on platelet shape change, aggregation and granule secretion induced by ADP or thrombin (Haaland and Holmsen, 2011). Epinephrine alone can also induce shape changes and platelet aggregation but with a much slower time-frame and to a lesser degree compared with that by ADP. It has been shown that the epinephrine-induced shape change results in pseudopods without changing the main cell-body size (Milton and Frojmovic, 1984).
In addition to platelet agonists, shape changes can occur without apparent aggregation by various stimuli. Angiotensin II, an important hormone for hypertension and vascular remodeling (Crowley et al., 2006), causes a significant increase in median platelet volume (MPV) in platelet rich plasma in vitro and potentiates platelet shape changes induced by ADP and serotonin, indicating that angiotensin II and the related signal transduction pathway may be linked with shape changes (Jagroop and Mikhailidis, 2000). This was further corroborated by the attenuation of angiotensin II-induced MPV increase and U46619 (a thromboxane A2 analogue)-induced platelet shape changes by losartan (a selective angiotensin II antagonist) at its therapeutic concentrations for antihypertensive effects. Immunological activation is also associated with platelet shape changes. Opsonized zymosan A, an immunological stimulus, can induce platelet shape changes that accompany reactive oxygen species (ROS) generation and mitochondrial membrane hyperpolarization (Matarrese et al., 2009). Immunoglobulin G (IgG) antiplatelet antibody can also induce morphological changes, as can be observed under microscopy, as a disk-to-sphere transformation associated with the formation of bulbous, short surface projections or pseudopodia (Colman et al., 1983).
Pathologically high shear stress, as observed in various disease states such as atherosclerosis, cancer or stenotic area, can induce platelet shape changes (Nesbitt et al., 2009). Shear rate can increase significantly up to 10,000 s−1 in cardiovascular diseases. The normal shear rate is around 200 s−1. Platelets respond to blood vessel injury and start rolling through the interaction between the GP1b/IX complex and the von Willebrand factor (vWF). The shear stress induces the rolling platelets which tightly adhere to the vessel wall by interactions between integrin αIIbβ3 and surface matrices.
Exogenous stimuli
Many environmental toxicants and xenobiotics can induce platelet activation and cardiovascular diseases (Savouret et al., 2003; Abrescia and Golino, 2005; Kim et al., 2014). Methylmercury, a cardiovascular toxicant that can induce platelet aggregation and prime granular release by activating the prostaglandin synthesis pathway, also primes platelet shape changes (Macfarlane, 1981; Jennrich, 2013). Xenobiotics that can affect actin dynamics also induce morphological changes in platelets. Cytochalasin D, which interferes with actin polymerization, can induce platelet shape changes (Fox and Phillips, 1981). The microtuble disassembling drug, vincristine, induces the transformation of discoid platelets to rounded cells. Nanoparticles have gained a lot of attention due to their effects on platelets. Platelets tend to be more susceptible to nanoparticles compared to other cells with larger sizes. A variety of nanoparticles can induce cytoskeletal alterations and the aggregation of platelets. Carbon nanoparticles (Radomski et al., 2005), silver nanoparticles (Jun et al., 2011) and more recently, polystyrene latex nanoparticles (PLNPs) (Smyth et al., 2015) have been reported to induce platelet shape changes along with aggregation.
In cold environments, most eukaryotic cells either assume discoid shapes or do not change but platelets undergo deformation (Winokur and Hartwig, 1995). Chilling platelets (4 degree) in vitro results in an increase in volume and the formation of loose marginal microtubules as well as pseudopods. The shape changes are observed even at 0 degree or in ice water but are reversible. They can also be mediated by myosin phosphorylation, cytosolic calcium increase and actin filament elongation (Van Poucke et al., 2014).
PATHOLOGICAL IMPLICATION OF PLATELET SHAPE CHANGES AND CYTOSKELETON DYNAMICS
Platelets with morphological changes become hyper-reactive to agonists (Maurer-Spurej and Devine, 2001) and xenobiotics that can prime platelet shape changes can potentiate the platelet aggregation responses and ultimately thrombosis. Hyper-reactivity or prothrombotic traits in morphologically changed platelets can be explained by granule secretion. Platelet shape change can trigger granule secretion through actin-myosin contraction. After platelet shape change is completed, granules and organelles are centralized in the cells (Furman et al., 1993). Although it is not clearly defined whether granule secretion occurs in platelets through OCS or plasma membrane fusion, the movement of granules following platelet shape change is considered a hallmark of granule secretion. A couple of Rab proteins including Rab27 (Tolmachova et al., 2007), SNAREs (Ren et al., 2007) and Munc (Schraw et al., 2003) proteins are known to induce the process in platelets. In the mice cremaster muscle arteriole thrombosis model, platelets are integrated and thrombi are formed upon laser-induced injury. Interestingly, platelets are disaggregated and easily washed off by blood flow with mild injury, whereas tight thrombi can form in accordance with P-selectin expression after severe injury (Stalker TJ et al., 2014). This suggests that stable thrombus formation is related to the irreversible platelet shape change and subsequent α-granule secretion.
Platelet shape change and concomitant actin filament turnover can release activating molecules that are bound to the cytoskeleton as inactive forms. Integrin αIIbβ3 is retained by the actin cytoskeleton in resting platelets but is released to the cell surface during platelet activation. It provides a receptor site for fibrinogen as well as vWF and promotes further adhesion of platelets. Therefore, it could increase pathologic thrombosis if platelet αIIbβ3 is expressed through thrombogenic agent-driven shape change. Down-regulation of αIIbβ3 as witnessed in some inherent diseases like Glanzmann’s thrombasthenia (Nurden, 2006) or by antiplatelet agents or anticancer drugs can lead to bleeding, namely compromised thrombosis.
In order to understand the mechanism underlying the turnover of these functional receptors, cytoskeleton related regulation has to be discussed. OCS might play a key role in platelet shape change and spreading, as the OCS occupies 50% of the total plasma membrane (White and Clawson, 1980). Patients with the Budd-Chiari syndrome, a condition caused by the formation of a blood clot within the hepatic veins, display dilated OCS in their platelets (Dayal et al., 1995). In line with this, it has been reported that integrin αIIbβ3 is highly expressed in the membrane of the OCS (Escolar et al., 1989). Recently, Mountford et al. (2015) showed that PI3KC2α knockdown significantly increases shear stress-induced platelet adhesion to fibrinogen and the collagen surface. Interestingly, the PI3KC2α disrupted platelets display enlarged OCS, suggesting that the pathologic platelet shape change may enhance thromboembolism (Mountford et al., 2015).
THERAPEUTIC OPPORTUNITY FOR REGULATING CYTOSKELETON DYNAMICS AND PLATELET SHAPE CHANGES
The polymerization of actin filaments and tubulin has been explored as therapeutic targets for a variety of diseases including cancer, cardiovascular diseases and thrombosis over the years. Since the morphological changes of platelets are closely associated with the pathophysiologic states, modulation of the platelet cytoskeleton can be a good therapeutic approach to improve disease states. Semaphorins were originally identified to induce growth cone collapse in developing neurites. Many studies have revealed the involvement of semaphorins in angiogenesis, immune cell regulation, and organogenesis. Among 25 Semaphorin family members, Semaphorin 3A (Sema3A) was found to be produced and secreted by endothelial cells. To investigate its role in blood, Kashiwagi et al. (2005) applied Sema3A to platelets in various conditions and demonstrated the inhibitory effects of Sema3A in platelet aggregation, αIIbβ3 expression, granular secretion, adhesion, and spreading through its receptors on the cells. In platelets, sema3A inhibited agonist-induced Rac1 activation and phosphorylation of actin-depolymerizing protein, cofilin, and indeed, it reduced F-actin contents. Interestingly, they could not detect any effect on the major platelet inhibitory pathways including cAMP and cGMP contents or alterations in the intracellular Ca2+ concentration. Therefore, sema3A mediated platelet inhibition appears to be related to the modulation of actin polymerization and morphological changes. The authors proposed that sema3A supports the maintenance of circulating platelets in a quiescent state and that the sema3A signaling pathway could be a novel target for antiplatelet therapy.
Similarly, 3 semaphorins and 8 plexins, receptors of semaphorin, have been detected in platelets (Wannemacher et al., 2011). Their roles in platelet function and in cell to cell interaction remain to be elucidated, although studies on sema4D−/− mice have demonstrated that sema4D plays a positive role in thrombus formation in vivo and platelet aggregation in vitro (Zhu et al., 2007). Importantly, sema4D−/− mice did not show gross bleeding and therefore, the authors suggested that sema4D could be a promising drug target to limit excessive platelet activation without disrupting normal platelet function.
Recently, Estevez et al. (2013) investigated the role of LIM kinase 1 (LIMK1) in platelet activation and thrombosis. LIMK1 is one of the effectors of Rac1, which facilitates actin polymerization, mediated through the phosphorylation and deactivation of cofilin. In the FeCl3-induced arterial thrombosis model as well as the tail bleeding time analysis model, the LIMK1-null mice revealed increased thrombotic occlusion time, but no difference in bleeding time compared with the wild-type. GP1bIX signaling-induced actin polymerization was indeed reduced in platelets from KO mice and more importantly, cytosolic phospholipase A2 (cPLA2)-dependent thromboxane A2 (TXA2) generation was inhibited.
It should be clarified further whether the rearrangement of modified actin directly affects platelet function. Many attempts have also been made to answer the question by using inhibitors of actin polymerization such as cytochalasins (Lefebvre et al., 1993; Torti et al., 1996; Natarajan et al., 2000). The results are contradicting, however, depending on the concentration of drugs used. Cytochalasin treatment inhibited αIIbβ3 activation at high concentrations, but enhanced platelet activation at low concentrations (Bennett et al., 1999). Nevertheless, it has been reported that talin is essential for integrin activation in platelets by linking the integrin cytoplasmic tail to actin filaments, suggesting that the dynamics of actin filaments by agonists can be important for platelet activation (Tadokoro et al., 2003).
The generation of platelet microparticles (PMP) could be another important result of platelet cytoskeleton rearrangement and shape change. PMPs are found in both normal and disease states. PMPs are also proposed to play active roles in many diseases including rheumatoid arthritis (Knijff-Dutmer et al., 2002), breast cancer (Janowska-Wieczorek et al., 2006), and cardiovascular diseases (Nomura et al., 2003). A recent study on the protein compositions of PMPs using a proteomics approach has revealed that the characteristics of PMPs are determined by the activating agonist, suggesting that PMPs are generated in specific pathophysiological states (Milioli et al., 2015).
Investigating platelet cytoskeleton regulation also represents an opportunity for the development of novel therapeutic targets. Gelsolin has been recently suggested as one of the targets (Liu et al., 2013), because platelet gelsolin level is positively correlated with cardiovascular diseases (CVD). The Xiongshao capsule, a Chinese herbal medicine used to improve blood circulation, reduced platelet gelsolin and platelet activation markers, similar to the antiplatelet drug aspirin (Liu et al., 2013).
Previous study has shown that filamin A (FlnA) null platelets are rapidly removed from the circulation (Nakamura et al., 2011; Thon and Italiano, 2012). FlnA-null megakaryocytes (MKs) did not show many differences in the expression of vWF receptor components such as GP1bα, ADAM17, metalloproteinase-9 (MMP-9) compared with wild-type MKs. However, the number of MKs was increased by 2.5-5 fold in the KO. The authors suggested that this is because of the fast removal of large and fragile platelets containing GP1bα degradation products, increased ADAM17 and MMP-9 (Jurak Begonja et al., 2011). Together with another report (Kanaji et al., 2012), the researchers have clarified that FlnA and GP1bα play crucial roles in determining platelet size during thrombopoiesis.
CONCLUSIONS
Platelet shape changes have many important implications in pathophysiology and toxicology, therefore, understanding the underlying mechanism and the interaction with xenobiotics and thrombotic activators will illustrate the pathophysiology of thrombotic diseases and provide important insights into molecular drug targets. In the same vein, modulation of the targets related to platelet shape changes would provide a novel therapeutic opportunity that can satisfy unmet medical needs in thrombotic diseases and prevent toxicity from prothrombotic xenobiotics.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (NRF-2016R1C1B3009116).
REFERENCES
- Abrescia P, Golino P. Free radicals and antioxidants in cardiovascular diseases. Expert Rev Cardiovasc Ther. 2005;3:159–171. doi: 10.1586/14779072.3.1.159. [DOI] [PubMed] [Google Scholar]
- Bennett JS, Zigmond S, Vilaire G, Cunningham ME, Bednar B. The platelet cytoskeleton regulates the affinity of the integrin αIIbβ3 for fibrinogen. J Biol Chem. 1999;274:25301–25307. doi: 10.1074/jbc.274.36.25301. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Born GV, Dearnley R, Foulks JG, Sharp DE. Quantification of the morphological reaction of platelets to aggregating agents and of its reversal by aggregation inhibitors. J Physiol. 1978;280:193–212. doi: 10.1113/jphysiol.1978.sp012380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller BS. Foreword - A brief history of ideas about platelets in health and disease. In: Michelson AD, editor. Platelets. Academic Press; 2013. pp. xix–xliv. [DOI] [Google Scholar]
- Collins B, Hollidge C. Antithrombotic drug market. Nat Rev Drug Discov. 2003;2:11–12. doi: 10.1038/nrd966. [DOI] [PubMed] [Google Scholar]
- Colman RW, Nachmias VT, Cines DB, Schreiber AD. Effect of antiplatelet antibody on platelet shape change, volume, and morphology. Am J Physiol. 1983;244:H357–H361. doi: 10.1152/ajpheart.1983.244.3.H357. [DOI] [PubMed] [Google Scholar]
- Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim H-S, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JL, Molish IR, Rigmaiden M, Stewart G. Evidence for a role of myosin phosphorylation in the initiation of the platelet shape change response. J Biol Chem. 1984;259:9826–9831. [PubMed] [Google Scholar]
- Dayal S, Pati HP, Pande GK, Sharma P, Saraya AK. Platelet ultra-structure study in Budd-Chiari syndrome. Eur J Haematol. 1995;55:294–301. doi: 10.1111/j.1600-0609.1995.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Escolar G, Leistikow E, White JG. The fate of the open canalicular system in surface and suspension-activated platelets. Blood. 1989;74:1983–1988. [PubMed] [Google Scholar]
- Estevez B, Stojanovic-Terpo A, Delaney MK, O’Brien KA, Berndt MC, Ruan C, Du X. LIM kinase-1 selectively promotes glycoprotein Ib-IX-mediated TXA2 synthesis, platelet activation, and thrombosis. Blood. 2013;121:4586–4594. doi: 10.1182/blood-2012-12-470765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JE, Phillips DR. Inhibition of actin polymerization in blood platelets by cytochalasins. Nature. 1981;292:650–652. doi: 10.1038/292650a0. [DOI] [PubMed] [Google Scholar]
- Furman MI, Gardner TM, Goldschmidt-Clermont PJ. Mechanisms of cytoskeletal reorganization during platelet activation. Thromb Haemost. 1993;70:229–232. [PubMed] [Google Scholar]
- Haaland HD, Holmsen H. Potentiation by adrenaline of agonist-induced responses in normal human platelets in vitro. Platelets. 2011;22:328–337. doi: 10.3109/09537104.2011.551949. [DOI] [PubMed] [Google Scholar]
- Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118:1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig JH. Chapter 8 - The Platelet Cytoskeleton A2. In: Michelson AD, editor. Platelets. Academic Press; 2013. pp. 145–168. [DOI] [Google Scholar]
- Hartwig JH, Barkalow K, Azim A, Italiano J. The elegant platelet: signals controlling actin assembly. Thromb Haemost. 1999;82:392–398. [PubMed] [Google Scholar]
- Hartwig JH, Bokoch GM, Carpenter CL, Janmey PA, Taylor LA, Toker A, Stossel TP. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109:5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- Jagroop IA, Mikhailidis DP. Angiotensin II can induce and potentiate shape change in human platelets: effect of losartan. J Hum Hypertens. 2000;14:581–585. doi: 10.1038/sj.jhh.1001102. [DOI] [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Marquez-Curtis LA, Wysoczynski M, Ratajczak MZ. Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion. 2006;46:1199–1209. doi: 10.1111/j.1537-2995.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Jennrich P. The influence of arsenic, lead, and mercury on the development of cardiovascular diseases. ISRN Hypertens. 2013;2013:234034. doi: 10.5402/2013/234034. [DOI] [Google Scholar]
- Jun EA, Lim KM, Kim K, Bae ON, Noh JY, Chung KH, Chung JH. Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity. Nanotoxicology. 2011;5:157–167. doi: 10.3109/17435390.2010.506250. [DOI] [PubMed] [Google Scholar]
- Jurak Begonja A, Hoffmeister KM, Hartwig JH, Falet H. FlnA-null megakaryocytes prematurely release large and fragile platelets that circulate poorly. Blood. 2011;118:2285–2295. doi: 10.1182/blood-2011-04-348482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaji T, Ware J, Okamura T, Newman PJ. GPIbα regulates platelet size by controlling the subcellular localization of filamin. Blood. 2012;119:2906–2913. doi: 10.1182/blood-2011-08-376566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi H, Shiraga M, Kato H, Kamae T, Yamamoto N, Tadokoro S, Kurata Y, Tomiyama Y, Kanakura Y. Negative regulation of platelet function by a secreted cell repulsive protein, semaphorin 3A. Blood. 2005;106:913–921. doi: 10.1182/blood-2004-10-4092. [DOI] [PubMed] [Google Scholar]
- Kim M, Han CH, Lee MY. NADPH oxidase and the cardiovascular toxicity associated with smoking. Toxicol Res. 2014;30:149–157. doi: 10.5487/TR.2014.30.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klages B, Brandt U, Simon MI, Schultz G, Offermanns S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J Cell Biol. 1999;144:745–754. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knijff-Dutmer E, Koerts J, Nieuwland R, Kalsbeek-Batenburg E, Van De Laar M. Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum. 2002;46:1498–1503. doi: 10.1002/art.10312. [DOI] [PubMed] [Google Scholar]
- Kovacsovics TJ, Hartwig JH. Thrombin-induced GPIb-IX centralization on the platelet surface requires actin assembly and myosin II activation. Blood. 1996;87:618–629. [PubMed] [Google Scholar]
- Kuhn S, Geyer M. Formins as effector proteins of Rho GTPases. Small GTPases. 2014;5:e29513. doi: 10.4161/sgtp.29513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara M, Sugimoto M, Tsuji S, Matsui H, Mizuno T, Miyata S, Yoshioka A. Platelet shape changes and adhesion under high shear flow. Arterioscler Thromb Vasc Biol. 2002;22:329–334. doi: 10.1161/hq0202.104122. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, White J, Krumwiede M, Cohen I. Role of actin in platelet function. Eur J Cell Biol. 1993;62:194–204. [PubMed] [Google Scholar]
- Liu Y, Yin H, Jiang Y, Xue M, Guo C, Shi D, Chen K. Correlation between platelet gelsolin and platelet activation level in acute myocardial infarction rats and intervention effect of effective components of chuanxiong rhizome and red peony root. Evid Based Complement Alternat Med. 2013;2013:985746. doi: 10.1155/2013/985746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane DE. The effects of methyl mercury on platelets: induction of aggregation and release via activation of the prostaglandin synthesis pathway. Mol Pharmacol. 1981;19:470–476. [PubMed] [Google Scholar]
- Matarrese P, Straface E, Palumbo G, Anselmi M, Gambardella L, Ascione B, Del Principe D, Malorni W. Mitochondria regulate platelet metamorphosis induced by opsonized zymosan A-activation and long-term commitment to cell death. FEBS J. 2009;276:845–856. doi: 10.1111/j.1742-4658.2008.06829.x. [DOI] [PubMed] [Google Scholar]
- Maurer-Spurej E, Devine DV. Platelet aggregation is not initiated by platelet shape change. Lab Invest. 2001;81:1517–1525. doi: 10.1038/labinvest.3780365. [DOI] [PubMed] [Google Scholar]
- Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milioli M, Ibáñez-Vea M, Sidoli S, Palmisano G, Careri M, Larsen MR. Quantitative proteomics analysis of platelet-derived microparticles reveals distinct protein signatures when stimulated by different physiological agonists. J. Proteomics. 2015;121:56–66. doi: 10.1016/j.jprot.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Milton JG, Frojmovic MM. Adrenaline and adenosine diphosphate-induced platelet aggregation require shape change. Importance of pseudopods. J Lab Clin Med. 1984;104:805–815. [PubMed] [Google Scholar]
- Mountford JK, Petitjean C, Putra HW, McCafferty JA, Setiabakti NM, Lee H, Tonnesen LL, McFadyen JD, Schoenwaelder SM, Eckly A, Gachet C, Ellis S, Voss AK, Dickins RA, Hamilton JR, Jackson SP. The class II PI 3-kinase, PI3KC2α, links platelet internal membrane structure to shear-dependent adhesive function. Nat Commun. 2015;6:6535. doi: 10.1038/ncomms7535. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Stossel TP, Hartwig JH. The filamins: organizers of cell structure and function. Cell Adh Migr. 2011;5:160–169. doi: 10.4161/cam.5.2.14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan P, May JA, Sanderson HM, Zabe M, Spangenberg P, Heptinstall S. Effects of cytochalasin H, a potent inhibitor of cytoskeletal reorganisation, on platelet function. Platelets. 2000;11:467–476. doi: 10.1080/09537100020027842. [DOI] [PubMed] [Google Scholar]
- Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A, Jackson SP. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- Nomura S, Uehata S, Saito S, Osumi K, Ozeki Y, Kimura Y. Enzyme immunoassay detection of platelet-derived microparticles and RANTES in acute coronary syndrome. Thromb Haemost. 2003;89:506–512. [PubMed] [Google Scholar]
- Nurden AT. Glanzmann thrombasthenia. Orphanet J Rare Dis. 2006;1:10. doi: 10.1186/1750-1172-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S, Toombs CF, Hu YH, Simon MI. Defective platelet activation in Gαq-deficient mice. Nature. 1997;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- Ohlmann P, Eckly A, Freund M, Cazenave JP, Offermanns S, Gachet C. ADP induces partial platelet aggregation without shape change and potentiates collagen-induced aggregation in the absence of Gαq. Blood. 2000;96:2134–2139. [PubMed] [Google Scholar]
- Paul BZ, Daniel JL, Kunapuli SP. Platelet shape change is mediated by both calcium-dependent and -independent signaling pathways. Role of p160 Rho-associated coiled-coil-containing protein kinase in platelet shape change. J Biol Chem. 1999;274:28293–28300. doi: 10.1074/jbc.274.40.28293. [DOI] [PubMed] [Google Scholar]
- Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, Radomski MW. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146:882–893. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AK, Vaidyula VR, Bagga S, Jalagadugula G, Gaughan J, Wilhite DB, Comerota AJ. Effect of antiplatelet agents clopidogrel, aspirin, and cilostazol on circulating tissue factor procoagulant activity in patients with peripheral arterial disease. Thromb Haemost. 2006;96:738–743. [PubMed] [Google Scholar]
- Ren Q, Barber HK, Crawford GL, Karim ZA, Zhao C, Choi W, Wang CC, Hong W, Whiteheart SW. Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Mol. Biol. Cell. 2007;18:24–33. doi: 10.1091/mbc.E06-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzer M, Essler M. Lysophosphatidic acid-induced platelet shape change proceeds via Rho/Rho kinase-mediated myosin light-chain and moesin phosphorylation. Cell Signal. 2000;12:645–648. doi: 10.1016/S0898-6568(00)00108-X. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/S0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Sanderson HM, Heptinstall S, Vickers J, Losche W. Studies on the effects of agonists and antagonists on platelet shape change and platelet aggregation in whole blood. Blood Coagul. Fibrinolysis. 1996;7:245–248. doi: 10.1097/00001721-199603000-00034. [DOI] [PubMed] [Google Scholar]
- Sandmann R, Koster S. Topographic cues reveal two distinct spreading mechanisms in blood platelets. Sci Rep. 2016;6:22357. doi: 10.1038/srep22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savouret JF, Berdeaux A, Casper R. The aryl hydrocarbon receptor and its xenobiotic ligands: a fundamental trigger for cardiovascular diseases. Nutr Metab Cardiovasc Dis. 2003;13:104–113. doi: 10.1016/S0939-4753(03)80026-1. [DOI] [PubMed] [Google Scholar]
- Schraw TD, Lemons PP, Dean WL, Whiteheart SW. A role for Sec1/Munc18 proteins in platelet exocytosis. Biochem J. 2003;374:207–217. doi: 10.1042/bj20030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth E, Solomon A, Vydyanath A, Luther PK, Pitchford S, Tetley TD, Emerson M. Induction and enhancement of platelet aggregation in vitro and in vivo by model polystyrene nanoparticles. Nanotoxicology. 2015;9:356–364. doi: 10.3109/17435390.2014.933902. [DOI] [PubMed] [Google Scholar]
- Smyth SS, Whiteheart S, Italiano JEJ, Coller BS. Platelet morphology, biochemistry, and function. In: Kaushansky K, Beutler E, Seligsohn U, Lichtman MA, Kipps TJ, Prchal JT, editors. Williams Hematology. The McGraw-Hill Companies, Inc; 2010. pp. 1735–1814. [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin β tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Thon JN, Italiano JE., Jr Does size matter in platelet production? Blood. 2012;120:1552–1561. doi: 10.1182/blood-2012-04-408724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmachova T, Abrink M, Futter CE, Authi KS, Seabra MC. Rab27b regulates number and secretion of platelet dense granules. Proc Natl Acad Sci USA. 2007;104:5872–5877. doi: 10.1073/pnas.0609879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti M, Festetics ET, Bertoni A, Sinigaglia F, Balduini C. Agonist-induced actin polymerization is required for the irreversibility of platelet aggregation. Thromb Haemost. 1996;76:444–449. [PubMed] [Google Scholar]
- Van Poucke S, Stevens K, Marcus AE, Lance M. Hypothermia: effects on platelet function and hemostasis. Thromb J. 2014;12:31. doi: 10.1186/s12959-014-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemacher KM, Wang L, Zhu L, Brass LF. The role of semaphorins and their receptors in platelets: Lessons learned from neuronal and immune synapses. Platelets. 2011;22:461–465. doi: 10.3109/09537104.2011.561891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Clawson CC. The surface-connected canalicular system of blood platelets--a fenestrated membrane system. Am J Pathol. 1980;101:353–364. [PMC free article] [PubMed] [Google Scholar]
- Winokur R, Hartwig JH. Mechanism of shape change in chilled human platelets. Blood. 1995;85:1796–1804. [PubMed] [Google Scholar]
- Zheng Y, Adams T, Zhi H, Yu M, Wen R, Newman PJ, Wang D, Newman DK. Restoration of responsiveness of phospholipase Cγ2-deficient platelets by enforced expression of phospholipase Cγ1. PLoS ONE. 2015;10:e0119739. doi: 10.1371/journal.pone.0119739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, Tamagnone L, Wagner DD, Milla ME, Brass LF. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci USA. 2007;104:1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]